| pA2
online © Copyright 2003 The British Pharmacological Society |
004P
University of Surrey Summer Meeting June 2003 |
Cigarette
smoke inhibits the induction on NOSII activity in murine macrophages
stimulated with gram negative bacteria
|
Print abstract Search PubMed for: Fleet
MR
|
Macrophages produce nitric
oxide (NO) in response to many stimulants, including lipopolysaccharide
(LPS) (Shultz, et al., 1991). The production of NO is believed
to have bacteriostatic effects, preventing or limiting infection (Klebanoff,
1993). Bacterial infection is particularly important in chronic obstructive
pulmonary disease (COPD). These patients have impairment of their local
immune defence which allows bacterial pathogens to gain a foothold in
the lower respiratory tract (Sethi, 2000). Since the most important risk
factor for developing COPD is smoking, we have looked at the effect of
cigarette smoke on NO synthase (NOS) activity induced by Gram negative
bacteria or bacterial LPS by murine J774 macrophages.
Cigarette smoke was bubbled from four cigarettes (full strength Marlboro)
through 100mls of DMEM, (100% CSE solutions; Walters & Mitchell, 2003)
and the 'strength' determined by measuring nitrite levels using the Greiss
reaction (Bishop-Bailey, et al., 1997). Each solution was added
immediately to the cells. The cells were then stimulated with either Escherichia
coli (E. coli) (8.85*108 CFU/ml)
or LPS (1µg/ml). In some experiments the mixed NOS inhibitor, L-NG-nitro-L-arginine
(L-NAME; 1mM) or the selective NOSII inhibitor, 1400W (100µM), were
added to the cells. Since cigarette smoke contains NO and other oxides
of nitrogen, the nitrite values of 'dummy plates' identical, except devoid
of cells, were prepared for each protocol and subtracted from those produced
by plates containing cells. NOS activity was determined by nitrite release.
TNF![]() levels were measured
using ELISAs. Incubations were for 24hrs. None of the treatments shown
affect cell viability (measured by the conversion of MTT to formazan;
Bishop-Bailey et al., 1997).
levels were measured
using ELISAs. Incubations were for 24hrs. None of the treatments shown
affect cell viability (measured by the conversion of MTT to formazan;
Bishop-Bailey et al., 1997).
Cells stimulated with whole heat killed E. coli (8.85*107-2.82*109
CFU/ml) or LPS (0.1-100µg/ml) released detectable nitrite with respective
Emax values of 25.0± 8.6µM
and 21.7±10.8µM (n=9 wells from 3 experimental days). Nitrite
release induced by either LPS (0.1µg/ml) or E. coli (8.85*108CFU/ml)
was respectively inhibited by either L-NAME (by 69.2±5.8% &
49.6±3.2%) or 1400W (by 82.1 ±2.4% & 85.8±1.5%),
(n=3-6). CSE caused concentration dependent inhibitions of nitrite release
induced by either LPS or E. coli (Fig.1).
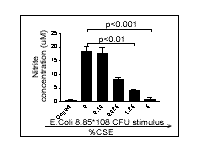
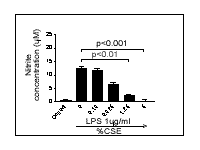
Figure 1: E. coli (A) or
LPS (B) stimulate nitrite release which is inhibited by CSE. The data
shown is the mean ± s.e.m. for n= 9 wells from n=3 experimental
days. All statistics shown are a One-way ANOVA with Dunnett's multiple
comparison test.
Similarly, J774 macrophages released increased levels of TNF![]() after
stimulation with either LPS or E. coli (basal 0±84.6pg/ml;
plus LPS, 4,465±2,445pg/ml; plus E. coli, 14,727±209pg/ml).
LPS induced TNF
after
stimulation with either LPS or E. coli (basal 0±84.6pg/ml;
plus LPS, 4,465±2,445pg/ml; plus E. coli, 14,727±209pg/ml).
LPS induced TNF![]() release
was inhibited by smoke extract (by 82.8±23% at 5% CSE, n=9). By
contrast, E. coli induced TNF
release
was inhibited by smoke extract (by 82.8±23% at 5% CSE, n=9). By
contrast, E. coli induced TNF![]() release was not affected by CSE (5%), (111±27% of control, n=9).
release was not affected by CSE (5%), (111±27% of control, n=9).
It is possible that the inhibitory effect of cigarette smoke on macrophage
immune function contributes to bacterial colonisation in the lungs in
patients with COPD.
Acknowledgement: This work was supported by the M.R.C.
Bishop-Bailey, D et al. (1997). Br. J. Pharmacol., 121,
125-133.
Klebanoff, S.J. (1993). Free Radic. Biol. Med., 14, 351-360.
Sethi,S. (2000). Chest, 117, 286S-291S.
Shultz, P.J. et al. (1991). Am. J. Phsiol, 261, F600-F606.
Walters M.J. & J.A. Mitchell (2003). BPS presentation (January
2003).