| pA2
online © Copyright 2003 The British Pharmacological Society |
005P
University of Surrey Summer Meeting June 2003 |
| Gram
positive and Gram negative bacteria synergise to cause nitric oxide
synthase induction in murine macrophages J. Anandarajah*, M.R. Fleet*, M.J. Walters*, P.E. Belcher*, S. Sriskandan1; J.A. Mitchell*.*Unit of Critical Care, The National Heart and Lung Institute, Imperial College, London, SW3 6LY, UK. 1Dept Infectious Diseases, Imperial College, Hammersmith Hospital W12 0HS. |
Print abstract Search PubMed for: Anandarajah
J |
The induction of nitric oxide
synthase II (NOS II, iNOS) and subsequent production of free radical gas
NO is an important anti-infectious mechanism of innate immunity, in which
macrophages play a significant role (Trajkovic, 2001). Murine macrophages
exposed to lipopolysaccharide (LPS), a component of Gram negative bacteria,
release large amounts of NO which contributes to their bacteriocidal activity
(Di Rosa et al, 1990). However the role of NO in the cytolysis
of Gram positive organisms is unknown. Furthermore, we have recently shown
that by contrast to LPS or Gram negative Escherichia.coli, Gram
positive Staphylococcus aureus does not induce murine macrophages
to release NO, despite stimulating TNF![]() release (Belcher et al, 2003). Components of S. aureus.
(peptidoglycan G and lipoteichoic acid) have been shown to act synergistically
with LPS to induce the release of a number of cytokines from murine and
human macrophages (Wang et al, 2001). However, the nature of any
interaction between whole S. aureus and LPS is not well understood.
release (Belcher et al, 2003). Components of S. aureus.
(peptidoglycan G and lipoteichoic acid) have been shown to act synergistically
with LPS to induce the release of a number of cytokines from murine and
human macrophages (Wang et al, 2001). However, the nature of any
interaction between whole S. aureus and LPS is not well understood.
Murine derived J774 macrophages were cultured in 96-well plates as described
previously (Mitchell et al., 1992) and stimulated for 24hrs with
LPS (0.01 to 10µg/ml), heat killed S. aureus (1.21 x 108
to 1.21 x 1010 cfu/ml), or LPS
plus S. aureus. In some experiments the NOS inhibitor L-NG-nitro-L-arginine
(L-NAME; 1mM) or D-NAME (1mM) was added to the cells just prior to LPS
or S. aureus respectively. NO was measured by the Greiss reaction.
LPS induced nitrite formation in a concentration dependant manner (Figure
1). Nitrite release was inhibited by L-NAME (by 82.3±4.3% at 10µg/ml
LPS; n=12) but not D-NAME (18.2±6.9%). Whole, heat killed S. aureus
did not induce nitrite release from J774 cells at concentrations up to
1.21 x 1010 cfu/ml.
However, at
1.21 x 109 CFU, S. aureus synergised with
LPS to cause an increase in nitrite release by J774 cells (Figure 1).
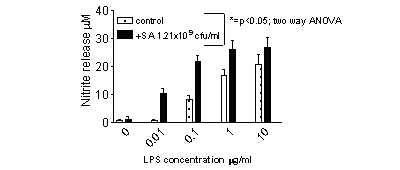
Figure
1; S. aureus and LPS synergise to induce nitrite release from J774 macrophages.
The figure shows the mean±s.e.m. for n=12 wells from 4 separate
experimental days. * represents significant differences between cells
treated with or without S. aureus (p<0.05; two-way ANOVA)
Here we show that whilst LPS clearly induces NOS activity and NO release
by murine macrophages, S. aureus has no effect. This observation
is consistent with our understanding that Gram positive and Gram negative
bacteria activate different pathways in the innate immune response (Gottar
et al. 2002). However, we also show a profound synergistic relationship
between S. aureus and LPS. This data suggests that where Gram positive
infections co-incide with sub clinical levels of Gram negative bacteria
or LPS, macrophages are activated via both pathways.
Belcher PE et al., BJA (2003) 90(4) 540P.
Di Rosa et al.,. Biochem Biophys Res Commun. (1990) 15;172(3):1246-52.
Gottar M et al., Nature (2002) 11;416(6881):640-4.
Mitchell JA et al., Mol Pharmacol. (1992) 41(6):1163-8.
Trajkovic V, Curr Drug Metab. (2001) 2 (3):315-29.
Wang JE et al., Shock (2001) 16(3): 178-82.
This work was supported by grants from the MRC and BHF.