| pA2
online © Copyright 2003 The British Pharmacological Society |
007P
University of Surrey Summer Meeting June 2003 |
|
Induction of NOSII and COX activity in primary cultures of murine smooth muscle cells
R. Jimenez,
T.D. Warner, P.E. Belcher, I. Vojnovic & J.A. Mitchell. The
William Harvey Research Institute, Barts and the London, Charterhouse
Square, London EC1M 6BQ and Unit of Critical Care, The National
Heart and Lung Institute, Imperial College, London, SW3 6LY, UK. |
Print abstract Search PubMed for: |
Nitric oxide
(NO) and prostaglandins are key mediators of cardiovascular health and
disease. In healthy vessels NO is produced in the endothelium via the
actions of constitutively expressed NOSIII. Similarly, prostaglandins
(principally prostacyclin and PGE) are also released by the endothelium
of healthy blood vessels, this time via the actions of constrictively
expressed cyclo-oxygenase (COX)-1 (Mitchell and Warner, 1999). However,
when blood vessels are damaged or exposed to inflammatory insult, inducible
forms of both NOS (NOSII) and COX (COX-2) are expressed in the underlying
vascular smooth muscle cells (Mitchell and Warner, 1999). This phenomenon
has been demonstrated in isolated vascular smooth muscle cell cultures
from laboratory animals (e.g. rats; Jourden et al., 1999) and from humans
(Bishop-Bailey et al., 1998). However, to our knowledge the relationship
between NOS and COX expression in primary cultures of mouse vascular smooth
muscle has not been studied. Because of the increasing availability of
genetically modified mouse models used experimentally we have investigated
the ability of cells grown from mouse aorta to express increased NOS and
COX activity after exposure to a number of inflammatory insults.
Mouse aorta was collected from C57/Bl6 mice cleared of connective tissue,
cut into pieces and placed in DMEM containing 20% fetal calf serum, penicillin
(100U/mL-1), streptomycin (100µg/mL-1),
amphotericin (2.5µg/mL-1) and non-essential
amino acids. Tissue was then placed into cultured and vascular smooth
muscle grown by standard explant (Bishop-Bailey et al., 1998).
After 3-5 passages, cells were grown to confluence in 96-well plates with
serum being withdrawn for 24 h prior to experimentation. All treatments
were made in DMEM containing 10% FCS and incubation maintained for 48h.
Under basal culture conditions, murine vascular smooth muscle cells release
low or undetectable levels of either NO (measured by the Griess reaction;
Bishop-Bailey et al., 1997; A) or PGE2
(measured by radioimmunoassay; Mitchell et al., 1993, B). However
after stimulation with either TNF![]() (100ng/ml), IL-1ß (10ng/ml) or LPS (1µg/ml) cells release
increased levels of both autocoids (Figure 1).
(100ng/ml), IL-1ß (10ng/ml) or LPS (1µg/ml) cells release
increased levels of both autocoids (Figure 1).
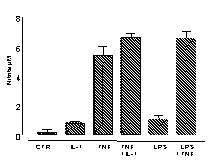
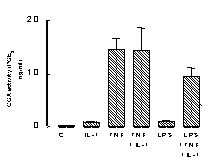
Data is mean
±s.e.m. for n=3 determinations, similar data was obtained using
cells cultured from a separate aorta.
Here we show that primary cultures of mouse vascular smooth muscle cells
can be maintained in culture and stimulated to release increased levels
of NOS and COX products, most likely via the NOSII and COX-2 pathways
respectively. This model is important because it will enable us to compare
responses in vascular tissue cultured from genetically modified mice.
This work was supported by a Fellowship grant from the Spanish Government
and grants from the Medical Research Council, the Joint Research Board
of St. Bartholomew's Hospital, and The William Harvey Research Foundation.
Bishop-Bailey D, Larkin SL, Williams TJ et al., (1997), Brit.
J. Pharmacol. 121: 125-133.
Bishop-Bailey D et al., (1998) Arterio. Thromb. Vasc. Biol.,
18: 1655-1661.
Jourdan KB, et al., (1999) Am J Respir Cell Mol Biol 21:
105-110.
Mitchell JA et al., (1993) Proc. Natl. Acad. Sci.,. 90:
11693-11697.
Mitchell JA & Warner TD (1999), Br. J. Pharmacol., 128:
1121-1132.