| pA2
online © Copyright 2003 The British Pharmacological Society |
014P
University of Surrey Summer Meeting June 2003 |
The
role of tyrosine residues at the mouse 5-HT3A
receptor lihand
binding site investigated by unnatural amino acid mutagenesis
|
Print abstract Search PubMed for: |
The 5-HT3
receptor (5-HT3R) is a member of the
Cys-loop family of ligand-gated ion channels and shares a high degree
of homology with nicotinic acetylcholine, GABAA/C,
glycine and GluCl receptors. Previous data has shown that the amino acids
involved in ligand binding comprise six non-contiguous loops (A-F). Tyrosine
residues from binding loops C (Y234) and E (Y141, Y143 and Y153) are important
for ligand binding and/or receptor gating transitions in the 5-HT3R
(Price et al. 2001, Venkataraman et al. 2002). To characterise
the role of these residues further, we have used in vivo nonsense suppression
to incorporate unnatural amino acids site-specifically in 5-HT3Rs
expressed in Xenopus oocytes.
Mutant mouse 5-HT3AsR subunits with TAG
codons in place of tyrosine codons were made using the Kunkel (1985) method,
subcloned into pGEMHE (Reeves et al., 2001), and then used for
in vitro transcription reactions (Ambion). Unnatural amino acids coupled
to the dinucleotide dCA were chemically synthesised, enzymatically ligated
to 74-mer THG73 tRNACUA (Nowak et al., 1998) and co-injected with mRNA into Xenopus oocytes.
Voltage clamp recordings were performed 18-36h post-injection, in some
cases on Opus Express (Axon) and pEC50
values calculated using the Hill equation (Table 1).
Table 1. pEC50s ± s.e. mean (n=3)
for 5-HTR3s mutated at positions 141,
143, 153 and 234. NR = no response, - = not attempted. Bold = significantly
different (P<0.01; ANOVA, Dunnett post test) to Tyr (WT).
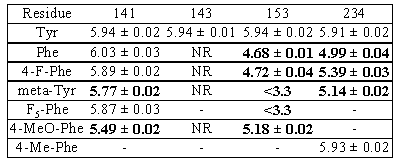
The results indicate that
the -OH groups of Y143 and Y153 are critical for binding and/or function,
whilst that of Y141 is not, as aromatic substitutions are well tolerated.
Aromatic substitutions of Y234 increase 5-HT EC50s
only modestly, but substituents at the 4 position seem to be advantageous.
These data provide support for a homology model of the 5-HT3R
extracellular domain (Reeves et al., 2003).
Kunkel (1985) Proc. Natl. Acad. Sci. U.S.A. 82 (2):488-492.
Nowak et al. (1998) Methods Enzymol. 293: 504-529.
Price et al. (2001) Br. J. Pharmacol. 134: 143.
Reeves et al. (2001) J. Biol. Chem. 276 (45): 42035-42042.
Reeves et al. (2003) Biophys. J. 84: 2338-2344.
Venkataraman et al. (2002) BMC Biochem. 3 (1):15.
Supported by the Wellcome Trust (SCRL), MRC (KLP), and NIH grants NS-11756
(HAL) and NS-34407 (DAD).