| pA2
online © Copyright 2003 The British Pharmacological Society |
025P
University of Surrey Summer Meeting June 2003 |
|
W Carey, E Clark, S Warrington, M Boyce, W Kerns1, P Fishman1, I Cohn1, M Silverman1& K Fong2 HMR, Central Middlesex Hospital, London, 1CanFite BioPharma Ltd, Israel, & 2Calvert Laboratories Inc, USA. |
Print abstract Search PubMed for: |
CF101 (IB-MECA,
methyl 1-[N6-(3-iodobenzyl)-adenin-9-yl] -ß-D-ibofuronamide, MW
510.29), is a selective A3
adenosine receptor agonist.We compared single oral doses
of CF101 solution (1, 5 and 10 mg in 30% Cremophor RH40) with placebo
with respect to safety, tolerability, pharmacokinetics (PK) and pharmaco-dynamics
(PD) in 3 groups of 5 (4 on active drug and 1 on placebo) healthy young
men in a double-blind study. We took blood samples before and after dosing,
to assay plasma CF101, serum G-CSF, GM-CFC (granulocyte-macrophage colony
forming cells) and CD34+ cells, and neutrophils. The study was approved
by a local ethics committee.
CF101 displayed first order kinetics, and concentrations were proportional
to dose (Table 1). Tmax (time
to peak concentration, Cmax)
was short. Half-life (t½)
and apparent plasma clearance (CL/F) were independent of dose.
Table 1: mean (SD) PK parameters (n=4 for all groups).
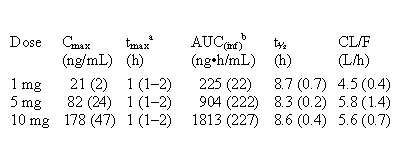
a
median (range) b Area under concentration-time curve, to infinity
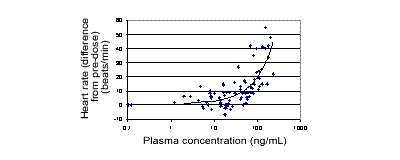
Single oral doses of CF101 up to 5 mg were safe, but, compared with placebo,
CF101 increased mean heart rate (Figures 1 and 2). There was no important
change in BP. Also, the 10 mg dose caused nausea and vomiting in 2 subjects.
At 4-8 h after dosing, CF101 caused a dose-related increase in peak neutrophil
count (10 mg group: median 11.34 x 109/L
(range 6.77-12.63) v placebo 4.40 x 109/L
(2.34-8.88); the neutrophil count returned to baseline by 24 h. There
were no significant changes in plasma G-CSF concentration, or GM-CFC or
CD34+ cell counts.
Figure 1: Mean semi-recumbent heart rate after CF101 (n=4)
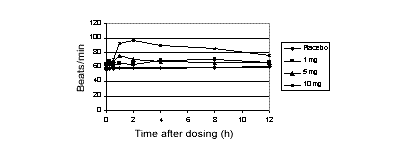
Figure 2: Change in heart rate (beats/min) with plasma CF101.
In conclusion, 10 mg CF101 was not tolerated because of increases in heart rate, and nausea and vomiting; the maximum tolerated dose was 5 mg.