| pA2
online © Copyright 2003 The British Pharmacological Society |
026P
University of Surrey Summer Meeting June 2003 |
| Repeated oral doses of CF101, a new adenosine A3 receptor agonist, in healthy young men
|
Print abstract Search PubMed for: Van
Troostenburg A
|
In single doses
up to 5 mg orally, CF101 was safe and well tolerated, but a 10 mg dose
increased heart rate and caused nausea and vomiting (Carey et al,
2003).
We compared repeated oral doses of CF101 suspension (2, 3, 4, and 5 mg
in 0.5% methyl cellulose, 12-hourly for 7 days) and placebo with respect
to safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD)
in 4 groups of 7 (5 on active drug and 2 on placebo) healthy young men
in a double-blind, parallel group study. We took frequent blood samples
on Days 1 and 7 to assess PK of CF101. The study was approved by a local
ethics committee.Plasma concentrations of CF101 were dose proportional,
and the pharmacokinetics did not change after repeated dosing.
Table 1: Mean (SD) plasma PK parameters after repeated 12-hourly doses
of CF101 (Day 7) (n=5 in each group).
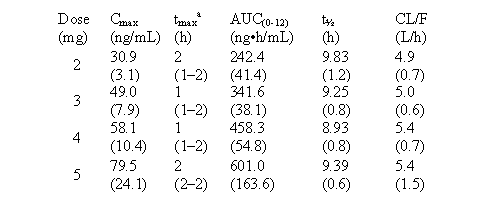
a
median (range) NB - see Carey et al for definitions.
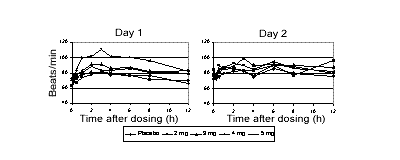
There was a weakly dose-dependent increase in mean heart rate on Day 1.
After 5 mg CF101, mean semi-recumbent heart rate increased by a maximum
of 22 beats/min (6 h post-dose), and 40 beats/min standing (3 h post-dose).
On Day 7, the effect of CF101 on heart rate was attenuated.
(Figure 1).Figure 1: Standing heart rate on Days 1 and 7.
There was a modest
dose-dependent increase in absolute neutrophil count on Day 1, but not
on Day 7. Median peak neutrophil count after 5 mg CF101 was 5.67 x 109/L
at 8 h post-dose on Day 1.
Repeated oral doses of up to 4 mg CF101 were safe and well tolerated,
but the 5 mg CF101 dose was less well tolerated. Most of the adverse events
were after that dose: nervous system disorders (headache and 'drowsiness')
were most common. 2 adverse events were vascular disorders (hot flushes
and dizziness on standing). Tolerance developed to the effect on heart
rate.
CF101 was safe and well tolerated at the 4 mg 12-hourly dose level, and
merits further clinical studies.~
W Carey, E Clark, S Warrington et al, this meeting.