| pA2
online © Copyright 2003 The British Pharmacological Society |
061P
University of Surrey Summer Meeting June 2003 |
|
The effects of chemical vagotomy of leptin-induced inhibition of food intake
|
Print abstract Search PubMed for: |
We have recently
reported that i.p., but not s.c., administration of leptin produces a
short lasting decrease in food intake in hungry rats and suggested that
leptin released from the stomach during a meal may play a role as a short-term
peripheral satiety factor (Patel et al., 2001, 2002). The study
was undertaken to investigate whether i.p. administered leptin activates
vagal afferents to signal the brain to inhibit food intake.
Chemical vagotomy was carried out using a slightly modified method described
previously (Brzozowski et al., 1996). Male Wistar rats (n=6; b.
wt. 320 -340 g) were pretreated with atropine sulphate (1 mg kg-1,
s.c.) and were anaesthetised with Equithesin daily on 3 consecutive days.
Following induction of anaesthesia, the animals were slowly infused i.p.
with the following doses of capsaicin: 25 mg kg-1
(day 1), 50 mg kg-1 (day 2) and 50 mg
kg-1 (day 3). Control rats (n=6) underwent
the same experimental procedure, but were injected with vehicle, instead
of capsaicin. The success of the c-fibre lesion of the afferent vagus
was confirmed by (a) the absence of abdominal constrictions to i.p. administration
of 0.01% acetic acid, and (b) the attenuation of the hypophagic effect
of i.p. administration of cholecystokinin (2 and 4 µg kg-1).
Although it is possible that capsaicin treatment also affected other sensory
nerves besides vagal afferents, we tried to minimise such collateral damage
by administering the capsaicin by the i.p., rather than the s.c. route,
so that the main effects would be on the gastric vagal afferents. Two
weeks later, the rats in both groups were fasted for 21h and injected
i.p. with either vehicle or leptin (25 µg kg-1)
immediately prior to being placed singly in experimental cages. The amount
of food consumed by each animal was measured at 15, 30 and 60 min. A cross-over
design was used with each rats receiving both treatments; 3 days separated
successive trials.
The results are shown in the Fig. 1. Control rats displayed a reduction
in cumulative food intake during the first 15 and 30 min after i.p. administration
of leptin compared with vehicle treatment. By contrast, rats that had
been treated with capsaicin did not display a reduction in food intake
after i..p. administration of leptin. The results indicate that chemical
vagotomy with capsaicin abolishes the inhibitory action of leptin (25
µg kg-1 i.p) on food intake and
suggests that the hypophagic effect is dependent on intact vagal afferent
nerves. The recent demonstration that leptin receptors are present on
vagal afferents (Buyse et al., 2001) lend further credence to the
hypothesis that leptin released from the stomach during a meal stimulates
vagal afferents to signal the brain to produce satiety.
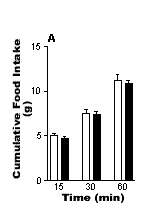
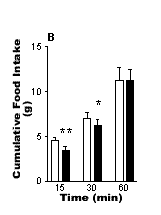
Fig 1. The effects of i.p. administration of leptin (25 mg kg-1) on food
intake in (A) chemically vagotomised rats (n=6) and (B) control (sham-treated)
rats that were fasted for 21h. ![]() Saline
Saline ![]() Leptin. Vertical
lines represent + s.e.mean **P<0.01, *P<0.01 (paired
t-test).
Leptin. Vertical
lines represent + s.e.mean **P<0.01, *P<0.01 (paired
t-test).
Brzozowski, T. et al. (1996) Digestion 57, 424 -
432.
Buyse, M. et al. (2001) Eur. J. Neurosci., 14, 64
- 72.
Patel, J.D. et al. (2002) Br. J. Pharmacol. 134,
118 P.
Patel, J.D. et al. (2001). Pharmacology Vancouver 2001,
1-106P.