| pA2
online © Copyright 2003 The British Pharmacological Society |
019P
University
of Manchester Autumn Meeting September 2003 |
|
Noladin ether attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a novel Gi/o linked cannabinoid receptor
|
Print Abstract Search PubMed
for: |
We have previously reported that noladin ether inhibits capsaicin-sensitive sensory neurotransmission via a non-CB1/CB2 mechanism in the rat mesenteric arterial bed (Duncan et al., 2002). In this study, we investigated whether the action is Gi/o receptor mediated, and whether noladin ether inhibits the capsaicin-induced calcium response in dorsal root ganglion (DRG) cells. HU210 has been previously reported to attenuate this response via CB1 receptors (Millns et al., 2001).Perfused rat mesenteric arterial beds were set up as described by Ralevic & Kendall, (2001).
Preparations were preconstricted with endothelin and methoxamine. Electrical field stimulation (EFS) was applied (8 & 12Hz; 60V, 0.1ms, 30s), 1µM noladin ether was added (40 min) then EFS repeated. PTX pretreatment was 10mg kg-1 (i.p.), 48 h prior to experiment. Data are expressed as mean±s.e.m. and analysed by ANOVA with Tukey's post-hoc test or by Student's unpaired t test. The DRG cells were cultured and processed as described by Lindsay (1998). Intracellular Ca2+ concentrations ([Ca2+]i) in individual neurones (23-26 cells) were calculated as the ratios of peak fluorescence intensities at excitation wavelengths of 340 & 380 nm. DRG neurones were superfused (2ml min-1) with capsaicin (100nM) for 60 s followed by a 45 min washout. Noladin ether (1 µM) was then added for 4 min, capsaicin in combination with noladin ether (1 µM) was then added for 60 s. After a period of 45 min washout, capsaicin alone was added. Responses were calculated as a percentage of the initial capsaicin response.
In the PTX-pretreated perfused
mesenteric beds the inhibitory actions of noladin ether on field-stimulation
induced sensory neurogenic relaxation were abolished. In control tissue
the 8Hz response was reduced from 39.0±4.4% to 22.9±6.1%,
and 12Hz reduced from 35.1±4.9% to 21.0±3.1% (n=10, P<0.01)
by noladin ether. There was no significant difference in the responses
in the pretreated tissues; 8Hz 36.2±5.9% and 40.0±6.1%,
12Hz 41.8±5.6% and 36.5±6.8% (n=8) in the absence and presence
of noladin ether respectively.
In DRG cells noladin ether alone induced no calcium response and had no
significant effect on the capsaicin-induced calcium response (Fig. 1).
In the presence and absence of noladin ether the capsaicin responses were
70.0±7.5% and 73.8±4.7% respectively.
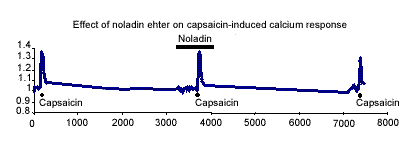
.Figure 1. Representative trace of the effect of 1mM noladin ether on the capsaicin-induced calcium response (100nM) in adult cultured rat dorsal root ganglion cells.
These data demonstrate that the inhibitory action of noladin ether on sensory neurogenic relaxation in rat mesenteric beds was abolished by PTX pretreatment, indicating that the site of action is a G-protein linked receptor. The preliminary results using the DRG cells indicate that noladin ether does not inhibit the capsaicin-induced calcium response. This is in agreement with our findings at peripheral sensory nerves that noladin ether is not acting via CB1 or VR1 receptors.
We are grateful to Servier for financial support.
Duncan, M. et al. (2002) Br. J. Pharmacol., 136, 13P.
Lindsay R.M. (1988) J. Neurosci., 8, 2394-2405.
Millns, P., et al. (2001) Br. J. Pharmacol., 132,
969-971.
Ralevic, V & Kendall, D.A. (2001) Eur. J. Pharmacol., 418,
117-25.