| pA2 online © Copyright 2004 The British Pharmacological Society |
025P
GKT, University of London Winter Meeting December 2003 |
|
Guanylate
cyclase receptors in human endothelial cells |
|
Natriuretic peptides (NPs), ANP BNP, and CNP, induce activation of guanylate cyclase (GC) receptors, GC-A and GC-B, with subsequent production of cGMP (Garbers et al., 1994). In the vascular bed, it is well-known that NPs increase cGMP production in smooth muscle cells (Sabrane et al., 2003). However, their effects on cGMP accumulation in endothelial cells is unknown. This investigation studied the effect of NPs on cGMP production and expression of GC-A and GC-B at mRNA level in human umbilical vein endothelial cells (HUVEC).
HUVEC were enzymatically isolated and seeded in 24 well plates at passage 1 or 2. Cells were stimulated with NPs for 3h in the presence of 500 µM IBMX, a phosphodiesterase inhibitor. Culture medium was collected to measure the cGMP production by ELISA (Amersham). Cell lysates were used to determine protein concentration. Expression of GC-A and GC-B mRNA was studied by RT-PCR. mRNA was isolated using Rneasy kit (Qiagen). Primers for GC-A and GC-B used for amplification have been previously described (Baldini et al., 2003). Control samples were tested for genomic and buffer contamination. Statistical analysis was performed using 1-way analysis of variance. Data are expressed as mean ± s.e.m.
At the lowest concentration (1 pM), NPs did not elevate cGMP formation: ANP (0.15±0.03 fmol cGMP/µg prot), BNP (0.13±0.05 fmol cGMP/mg prot) and CNP (0.12±0.01 fmol cGMP/µg prot; n=6 for each peptide). These levels are not significantly different from those observed in controls (0.16±0.02 fmol cGMP/µg prot, n=18; Figure 1).
In contrast at 10 nM, ANP (2.88±1.03 fmol cGMP/µg prot), BNP (5.79±2.43 fmol cGMP/µg prot), and CNP (15.26±2.58 fmol cGMP/µg prot) increased cGMP (n=6 and P<0.05 vs control for each peptide; Figure 1). CNP increased cGMP levels by 5.3- and 2.6-fold respectively compared to ANP and BNP (P<0.05 vs ANP and BNP).
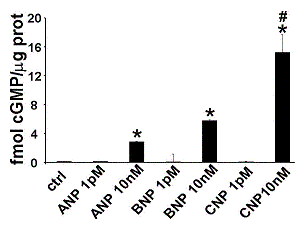
Figure 1: cGMP accumulation to NPs in HUVEC. * P<0.05 vs ctrl, # P<0.05 vs ANP and BNP 10 nM.#GC-A and GC-B were recognised as amplification products of 437 and 639 base-pairs, respectively. No products were observed in control samples. Both GC-A and GC-B mRNA were expressed in HUVEC.
For the first time, it is shown that ANP, BNP and CNP induced elevation in cGMP levels in HUVEC, which could be mediated via stimulation of GC-A and GC-B. Further pharmacological studies, using GC receptors antagonists, may explain the higher efficacy of CNP at equimolar concentration.
Baldini et al.,
(2003 ) J. Leucoc. Biol. 73: 502-510.
Garbers et al., (1994) J. Biol. Chem. 269: 30741-30744.
Sabrane et al., (2003) J. Biol. Chem. 278: 17963-17968.