| pA2 online © Copyright 2004 The British Pharmacological Society |
031P
GKT, University of London Winter Meeting December 2003 |
|
The FxR
ligand sr45023a (apomine) increases the neutrophil survival elements
(GM-CSF & G-CSF) but not the neutrophil chemokine |
|
Pulmonary hypertension of different origins, including that associated with COPD, is typified by structural changes in pulmonary vasculature and ongoing inflammation associated with neutrophils. Reversing or reducing the remodelling and inflammation are important therapeutic targets for the treatment of pulmonary hypertension. In the current study we have investigated the effects of the nuclear receptor ligand, SR45023A (Flach et al, 2000) which activates Farnesoid X receptors (FxR), on markers of inflammation in human pulmonary vascular smooth muscle cells (HPASMC).
Human pulmonary artery smooth muscle cells (HPASMC) were obtained and cultured in 96-well plates as described previously (Jourdan et al., 1999). Cell proliferation following incubation with foetal calf serum (FCS, 3 %) was determined by the incorporation of methyl-[3H]thymidine (Wort et al., 2001). Cytokine release was determined by ELISA following incubation (24 h) of cells with interleukin (IL) -1ß (1 ng ml-1) in the presence of SR45023A, or vehicle (0.1% DMSO) (Stanford et al., 2000).
SR45023A inhibited proliferation (A) increased GM-CSF (B) or G-CSF (C) and had no effect on IL-8 (D) release by stimulated HPASMC (n=9, cells cultured from 3 separate donors *=p<0.05 One-way ANOVA).
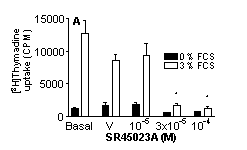
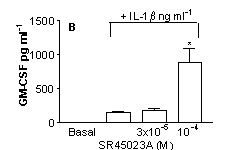
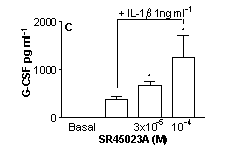
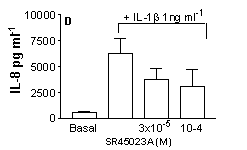
These data show, for the first time, a function of SR45023A in human lung cells and suggest that activation of FxR may result in a specific cellular response resulting in increased neutrophil survival without activation and illustrate the potential usefulness of FxR receptors as a therapeutic target in human disease, particularly those associated with immune deficiency disorders.
Flach J et al. (2000)
Biochem. Biophy Res. Comm. 270; 240-246.
Jourdan KB et al. (1999) Am J Respir Cell Mol Biol. 21,105-10.
Stanford S et al. (2000) Arterioscl. Thromb. Vasc. Biol., 20,835-838.
Wort, J.S. et al. (2001) Am. J. Resp. Cell Mol. Biol. 25,104-10.
This study was funded by an Industrial collaborative grant between the MRC and Yamanouchi. D B.B is a BHF Fellow.