| pA2 online © Copyright 2004 The British Pharmacological Society |
035P
GKT, University of London Winter Meeting December 2003 |
|
Expression
of COX-1, but not COX-2 or COX-3 - like immunoreactivity in human
blood vessels |
|
Cyclooxygenases (COXs) catalyze the conversion of arachidonic acid to prostaglandin H2, which serves as the common precursor for the synthesis of prostanoids. COX is expressed in two distinct isoforms. COX-1 is present constitutively in platelets and endothelial cells, whereas COX-2 is induced at sites of inflammation. The COX-1 and COX-2 proteins have a marked degree of homology (>60 %) but important differences exist between their enzymatic sites. These differences have allowed the development of selective inhibitors of the two forms (Mitchell and Warner, 1999). Recently, the presence of a third isoform, COX-3 (canine COX-3 and human aorta), has been suggested (Chandrasekharan et al., 2002). However, the levels of COX-1, COX-2 or the putative COX-3 enzyme in human cardiovascular tissue have not been compared directly.
Here, we have used western blotting with primary antibodies raised to the three forms of COX to investigate the relative levels of each isoform in human saphenous vein, internal mammary artery (from n = 4 patients) or radial artery (n = 1 patient). Human vessels were removed during standard coronary artery bypass surgery and immediately snap frozen in liquid nitrogen. Proteins were extracted using a mortar and pestle in HEPES buffer containing NP40 (0.3 %) and PMSF (10-3 M). 20 µg of protein was loaded on to SDS gels and western blots carried out using primary antibodies to COX-1 and COX-2 (from Cayman) or COX-3 (from Alpha diagnostic International) (Mitchell et al., 1993; Chandrasekharan et al., 2002 ).
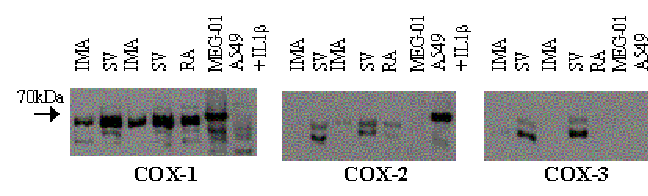 Figure
1. Western blot analysis of COX-1, COX-2 and COX-3 expression (70 kDa)
from fresh IMA (internal mammary artery) and SV (saphenous vein) of two
patients, RA (radial artery), human megakaryocytic cells (MEG-01 cells;
COX-1 expression control) and IL-1ß-stimulated human lung epithelial
cells (A549 cells; COX-2 expression control).
Figure
1. Western blot analysis of COX-1, COX-2 and COX-3 expression (70 kDa)
from fresh IMA (internal mammary artery) and SV (saphenous vein) of two
patients, RA (radial artery), human megakaryocytic cells (MEG-01 cells;
COX-1 expression control) and IL-1ß-stimulated human lung epithelial
cells (A549 cells; COX-2 expression control).
Only COX-1 was detected in all tissues examined (Figure 1). Densitometer analysis showed that COX-1 expression was higher in SV (23.5 ± 0.9 %; mean ± s.e.m., n = 4 patients) than in IMA (8.5 ± 0.4 %; mean ± s.e.m., n = 4 patients). Two proteins at 65 and 50 kDa were also recognized by both the COX-2 and the COX-3 antibodies in SV. Neither of our cell based control systems for COX-1 or COX-2 contained the 65 or 50KDa bands. These experiments show clearly that COX-2 is not a detectable feature of human blood vessels, even those donated by individuals with ongoing cardiovascular disease. These findings have implications for our understanding of perceived risk of cardiovascular events with the newer generation of COX-2 selective drugs.
Mitchell et al.
(1993) Proc. Natl. Acad. Sci.,. 90: 11693-11697.
Mitchell and Warner (1999) Br. J. Pharmacol., 128: 1121-1132.
Chandrasekharan et al. (2002) Proc. Natl. Acad. Sci., USA
99:13926-13931.
This work was supported by a Fellowship grant from the Spanish Government and grants from the Medical Research Council, the Joint Research Board of St. Bartholomew's Hospital, and The William Harvey Research Foundation.