| pA2 online © Copyright 2004 The British Pharmacological Society |
036P
GKT, University of London Winter Meeting December 2003 |
|
Characterisation
of cyclo-oxygenase activity in a human megakaryocyte cell line:
relevance to platelet COX-2 |
|
Cyclo-oxygenase (COX)-1 in platelets regulates the production of thromboxane (TX)A2 and thereby, platelet aggregation. Most recently clinical studies have suggested that COX-2 may be expressed in platelets following coronary artery bypass surgery (Weber et al, 2002). COX-2 is not normally expressed in most cells but is induced following exposure of cells or tissues to inflammatory cytokines. As platelets themselves have no nucleus, it is not immediately clear how they may come to contain COX-2. However, platelets are derived from megakaryocytes, which are nucleated. Here we have investigated the effects of COX-1 or COX-2 selective inhibitors on prostanoid production by control and cytokine-treated human megakaryocytes.
The megakaryocytic
cell line, MEG-01 was purchased from ATCC and cultured in 75 cm2
tissue culture flasks containing RPMI-1640 modified according to ATCC
to also contain: 10mM HEPES, 1mM sodium pyruvate, 4.5g/L glucose, and
supplemented with 10% fetal bovine serum. Cells were then transferred
to 96-well plates and grown to confluence at which time they were incubated
with a combination of IL1ß plus TNF![]() (10ng/ml for each) or vehicle for 24 hours. This was followed by incubation
with either vehicle (0.1% v/v DMSO), SC560 (COX-1 inhibitor; 10-7-10-4
M) or rofecoxib (10-7-10-4M)
for 1h followed by 30 min stimulation with arachidonic acid 30µM.
COX activity was determined as the accumulation of prostaglandin E2
(PGE2) or thromboxane B2
(TXB2) as measured by RIA (Mitchell et al., 1993).
(10ng/ml for each) or vehicle for 24 hours. This was followed by incubation
with either vehicle (0.1% v/v DMSO), SC560 (COX-1 inhibitor; 10-7-10-4
M) or rofecoxib (10-7-10-4M)
for 1h followed by 30 min stimulation with arachidonic acid 30µM.
COX activity was determined as the accumulation of prostaglandin E2
(PGE2) or thromboxane B2
(TXB2) as measured by RIA (Mitchell et al., 1993).
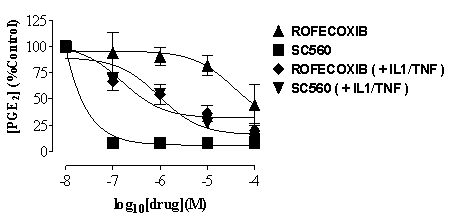
Fig. 1 COX activity
(PGE2) in MEG-01 cells shown as % of
control in cells cultured under control conditions (squares and right-way
triangles) or after stimulations with the combination of IL-1ß plus
TNF![]() (10ng/ml for each).
Data is mean ± s.e.m. for n=3 determinations.
(10ng/ml for each).
Data is mean ± s.e.m. for n=3 determinations.
'Control' cells released
detectable levels of TXB2 (1.8±0.3
ng/ml) or PGE2 (2.9±1.0 ng/ml).
This was increased following incubation with IL-1ß plus TNF![]() (TXB2 3.2±0.6 ng/ml; PGE2
9.3±4.0 ng/ml). Under control conditions the production of PGE2
and TXA2 were inhibited more potently
by SC560 than by rofecoxib (Figure 1). However, after IL1ß plus
TNF
(TXB2 3.2±0.6 ng/ml; PGE2
9.3±4.0 ng/ml). Under control conditions the production of PGE2
and TXA2 were inhibited more potently
by SC560 than by rofecoxib (Figure 1). However, after IL1ß plus
TNF![]() , SC560 and rofecoxib
were equipotent inhibitors of PGE2 and
TXA2 production. The expression and function
of COX isozymes and prostanoids in haematopoietic progenitors and lineages
remain largely unknown. This data demonstrates processes through which
platelets may express COX-2 and has important implications for our understanding
of the potential effects of COX-2 selective inhibitors in the cardiovascular
system.
, SC560 and rofecoxib
were equipotent inhibitors of PGE2 and
TXA2 production. The expression and function
of COX isozymes and prostanoids in haematopoietic progenitors and lineages
remain largely unknown. This data demonstrates processes through which
platelets may express COX-2 and has important implications for our understanding
of the potential effects of COX-2 selective inhibitors in the cardiovascular
system.
Weber et al.,
(2002), Brit. J. Haematology. 117:424-426.
Mitchell et al., (1993), Proc. Natl. Acad.Sci., 90:
11693-11697.
This work was funded by the British Heart Foundation.