| pA2 online © Copyright 2004 The British Pharmacological Society |
067P
GKT, University of London Winter Meeting December 2003 |
|
Telomere
length shortening in cultured internal mammary endothelial cells
from patients with coronary artery disease |
|
Telomeres, the TTAGGG tandem repeats at the ends of mammalian chromosomes, undergo attrition with each division of somatic cells. Their length is, hence, an indicator of both replicative history and replicative potential of the cells. When this potential is exhausted, cells enter a permanent non-dividing state, the replicative senescence. Cardiovascular risk factors increase endothelial cell (EC) turnover (Schwartz et al., 1977); we hypothesized that this could accelerate telomere loss, which could lead to premature replicative senescence.
To test this hypothesis, ECs were isolated from 8 human internal mammary arteries (5 males, 49 to 69 y/o and 3 females, 61 to 73 y/o) discarded during coronary artery bypass graft surgery. Cells were maintained in cultured and passaged until they reach replicative senescence, i.e. growth arrest. At each passage, DNA (5-20µg) was extracted and digested with Rsa I / Hinf I. Telomere restriction fragment length (RFL, bp) was measured by southern blotting (Chang et al., 1995) using a [32P]-[CCCTAA]4 probe. The mean RFL length was revealed by phosphor imaging.
Between the early
and the last passage of cultured EC obtained from 8 different patients,
telomere length significantly decreased, demonstrating telomere shortening
as days in culture increased (RFL = -1.03 1n(days) + 10.975 v=0.69, P
< 0.05). However, the rate of telomere shortening, i.e the loss of
RFL during the time elapsed between the first and the last passage (![]() RFL
/
RFL
/ ![]() Days, bp/day), varied
from 3 to 37 bp/day (Figure 1). Figure 1 shows that the maximal telomere
shortening was greater in EC that were maintained until growth arrest
in culture for the shortest time, suggesting that premature replicative
senescence occurred earlier in the EC of these patients. Accordingly,
neither telomere length nor the rate of telomere shortening was dependent
of the age of the donor (P > 0.05).
Days, bp/day), varied
from 3 to 37 bp/day (Figure 1). Figure 1 shows that the maximal telomere
shortening was greater in EC that were maintained until growth arrest
in culture for the shortest time, suggesting that premature replicative
senescence occurred earlier in the EC of these patients. Accordingly,
neither telomere length nor the rate of telomere shortening was dependent
of the age of the donor (P > 0.05).
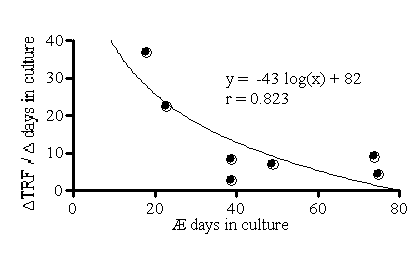
Figure 1: Rate of
telomere shortening in human EC.
In EC from atherosclerotic patients, culture duration time to reach replicative senescence is heterogeneous and independent of the age of the donor. Thus, EC from each individual possess a unique replicative potential. Premature senescence of human vascular EC could be due to cardiovascular risk factors, which accelerate EC turnover.
Chang et al. (1995),
Proc Natl Acad Sci USA, 92, 11190-4.
Schwartz et al. (1977), Circ Res, 41, 248-55.