| pA2 online © Copyright 2004 The British Pharmacological Society |
129P
GKT, University of London Winter Meeting December 2003 |
|
The effects
of chemical vagotomy on calcitonin gene related peptide (CGRP)-induced
inhibition of food intake in rats |
|
We have previously demonstrated that i.p. administration of CGRP produces a short lasting decrease in food intake in rats in a behaviourally specific manner (Price et al., 1998, 1999) and have suggested that the hormone released from the gut during a meal acts as a satiety factor. As CGRP is a 37 amino acid peptide, it is unlikely that it crosses the blood brain barrier to enter the CNS from the systemic circulation. It has been suggested that the peptide may signal the brain via vagal afferents to elicit its hypophagic effect. The present study was undertaken to investigate this possibility.
Chemical vagotomy was carried out as described previously (Patel & Ebenezer, 2003). Male Wistar rats (n=5; b. wt. 320 -340 g) were pre-treated with atropine sulphate (1 mg kg-1, s.c.) and were anaesthetised with Equithesin daily on 3 consecutive days. Following induction of anaesthesia, the animals were slowly infused i.p. with the following doses of capsaicin: 25 mg kg-1 (day 1), 50 mg kg-1 (day 2) and 50 mg kg-1 (day 3). Control rats (n=6) underwent the same experimental procedure, but were injected with vehicle, instead of capsaicin. The success of the c-fibre lesion of the afferent vagus was confirmed by the attenuation of the hypophagic effect of i.p. administration of cholecystokinin (2 and 4 µg kg-1). Two weeks later, the rats in both groups were fasted for 21h and injected i.p. with either vehicle or CGRP (10 µg kg-1) immediately prior to being placed singly in experimental cages. The amount of food consumed by each animal was measured at 15, 30 and 60 min. A cross-over design was used with each rat receiving both treatments; 3 days separated each trial. The data was analysed using the paired t-test.
The results are shown in Fig. 1. Control rats displayed a reduction in cumulative food intake during the first 15, 30 and 60 min after i.p. administration of CGRP compared with vehicle treatment. Likewise, rats that had been treated with capsaicin displayed a similar reduction in food intake after i..p. administration of GCRP. The results indicate that chemical vagotomy with capsaicin does not abolish the inhibitory action of CGRP (10 µg kg-1 i.p) on food intake and suggests that the hypophagic effect is not dependent on intact vagal afferent nerves. Recently, Lutz et al. (1998) have demonstrated that lesions of the area postrema/nucleus tractus solitarius in the brain stem attenuate the anorectic effect of CGRP in the rat. As this area of the CNS is poorly protected by the blood brain barrier, it is conceivable that these brainstem nuclei may be the sites at which the peptide acts to elicit its inhibitory effects on food intake following i.p. administration.
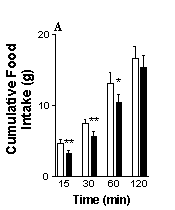
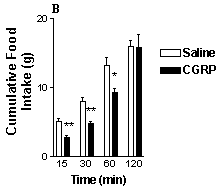
Fig 1. The effects of i.p. administration of CGRP on food intake in (A) chemically vagotomised rats and (B) control (sham-treated) rats.Vertical lines represent + s.e.mean **P<0.01, *P<0.05 (paired t-test).
Patel, J.D. &
Ebenezer, I.S. (2003) Br. J. Pharmacol. 140, 61P
Price, M.E. et al. (1999) Br. J. Pharmacol., 129, 148P
Price, M.E. et al. (1998) J. Physiol. (Lond)., 513, 8P.
Lutz, T.A. et al. (1998) Peptides, 19, 309 - 317.