| pA2 online © Copyright 2004 The British Pharmacological Society |
144P
GKT, University of London Winter Meeting December 2003 |
|
No evidence
for the presence of COX-3 in rat tissues |
|
Nonsteroid anti-inflammatory drugs (NSAIDs) inhibit the activity of cyclooxygenase (COX) -1 and COX-2. It has been suggested that there may be an additional product of the COX-1 gene, called COX-3, present in the brain (and other tissues) and particularly sensitive to inhibition by paracetamol and related drugs (Chandrasekharan et al., 2002). Here we have characterised COX enzymes present in a number of rat tissues by drug sensitivity and western blot analysis.
Male Wistar rats
(220-250 g) were anaesthetised with Inactin™ (120 mg kg-1,
i.p.) and blood removed via a cannula placed into the carotid artery.
Some blood was centrifuged to produce plasma and the rest aliquoted into
96-well plates. Animals were then killed and test tissues removed. Tissues
were finely chopped, except for aortae which were cut into rings, and
placed into 100 µl incubates of rat plasma. The test compounds,
naproxen (non-selective), paracetamol, rofecoxib (COX-2-selective) or
SC560 (COX-1-selective) (all 10-6 to
10-3 M) were then added and incubations
continued for 60 min. 50 µl aliquots were then removed ('basal')
and calcium ionophore (A23187, 50 µM) added. Incubations then continued
for a further 30 min before medium was removed ('stimulated'). As a measure
of prostanoid production the amounts of 6-keto-PGF1![]() (a marker of PGI2) or thromboxane
(Tx) B2 (a marker of TxA2)
in samples were determined by radioimmunoassay. The net production of
prostanoid by tissues in response to A23187 was determined as 'stimulated'
minus 'basal'.
(a marker of PGI2) or thromboxane
(Tx) B2 (a marker of TxA2)
in samples were determined by radioimmunoassay. The net production of
prostanoid by tissues in response to A23187 was determined as 'stimulated'
minus 'basal'.
Prostanoid formation
in the aorta, heart, lung and whole blood was inhibited by compounds with
the order of potency, SC560 > naproxen > paracetamol ![]() rofecoxib; in the brain and cerebellum the compounds were approximately
equipotent (Figure 1; n=4-6 for all). Western blot analysis demonstrated
expression of COX-1 in the aorta, heart, lung and whole blood and COX-1
and COX-2 in the cerebellum and brain (n=3 for all). No evidence was found
for expression of COX-3.
rofecoxib; in the brain and cerebellum the compounds were approximately
equipotent (Figure 1; n=4-6 for all). Western blot analysis demonstrated
expression of COX-1 in the aorta, heart, lung and whole blood and COX-1
and COX-2 in the cerebellum and brain (n=3 for all). No evidence was found
for expression of COX-3.
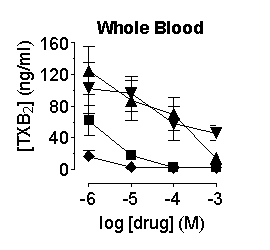
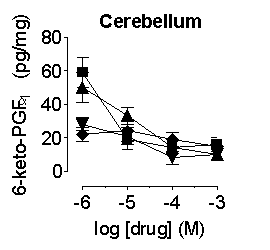
Figure
1. Inhibition of prostanoid formation by naproxen (![]() ),
paracetamol (
),
paracetamol (![]() ), rofecoxib
(
), rofecoxib
(![]() ) and SC560
(
) and SC560
(![]() ) in rat whole blood
and cerebellum.
) in rat whole blood
and cerebellum.
Our data show that in fresh tissues (i.e. avoiding the risk of enzyme induction) prostanoid formation in peripheral tissues is due to the activity of COX-1. In tissues from the CNS, prostanoid production is supported by both COX-1 and COX-2. There is no evidence in the rat for the presence of COX-3.
Chandrasekharan, N.V. et al. (2002). Proc. Natl. Acad. Sci. USA, 99, 13926-13931.
This work was supported by a grant from the William Harvey Research Foundation.