| pA2 online © Copyright 2004 The British Pharmacological Society |
193P
GKT, University of London Winter Meeting December 2003 |
|
T-cell recognition
of carbamazepine and carbamazepine metabolites |
|
T-cells are responsible for the initiating and effector arms of drug allergy. Using T-cell clones from allergic patients (Naisbitt et al., 2003a&b) we have shown that drugs are presented in a pathway that is MHC-restricted, but processing and metabolism independent. Despite this, the nature of the interaction between drug, MHC and the T-cell receptor is ill defined. T-cells from carbamazepine (CBZ) allergic patients proliferate with CBZ, CBZ metabolites (CBZ epoxide, 10-hydroxy CBZ) and oxcarbazepine (oxCBZ; a related drug). The aim of this project was to clone T-cells from a CBZ allergic patient and study whether presentation of CBZ and CBZ metabolites is due to cross reactivity at a single T-cell receptor or a heterogeneous T-cell response against both drug and metabolites. Computer modelling was used to visualise the preferred drug confirmations and how this relates to the T-cell response.
T-cell clones were generated by serial dilution and mitogenic restimulation (Naisbitt et al., 2003a). To test the specificity of the clones, T-cells (0.5x105) were incubated with B-cells as antigen presenting cells (APC; 0.1x105) in the presence of the test compounds (10-100µg/ml). After 48h, [3H] thymidine (0.5µCi) was added. To study the mechanism of presentation to T-cells: (1) APC were fixed with glutaraldehyde; (2) APC were pulsed with drug (50µg/ml) for 2h, washed and added to the proliferation assay in drug-free medium; and (3) glutathione (1mM), was added to the proliferation assay. Glutaraldehyde inhibits antigen processing, pulsed APC only present covalently bound CBZ and glutathione prevents covalent binding. Energy minimized models were generated with Cerius2 software on a Silicon Graphics workstation. After testing the specificity of 1200 T-cell clones, 24, 18, 32 and 4 clones proliferated in the presence of CBZ, CBZ epoxide, 10-hydroxy CBZ and oxCBZ, respectively (further experiments increased the number of oxCBZ clones). Clones were considered drug-specific when proliferation in incubations containing drug was greater than 2.5 fold higher than proliferation in control incubations. T-cell clones did not respond to pulsed APC, while the addition of glutathione and fixation of APC did not inhibit proliferation. Structure activity studies with 29 clones revealed 4 patterns of recognition. First, 13 (1 CBZ, 5 CBZ epoxide & 7 oxCBZ clones) clones were highly specific and only proliferated with one compound; secondly, 5 (3 CBZ epoxide & 2 oxCBZ clones) clones were broadly cross-reactive; thirdly, 6 (4 CBZ & 2 CBZ epoxide clones) clones proliferated with CBZ and CBZ epoxide (fig. 1a); and finally, 5 (4 10-hydroxy CBZ & 1 oxCBZ clones) clones proliferated with 10-hydroxy CBZ and oxCBZ (fig. 1b). Energy minimized modelling grouped CBZ and CBZ epoxide and 10-hydroxy CBZ and oxCBZ according to 3-dimensional orientation (Fig. 1c).
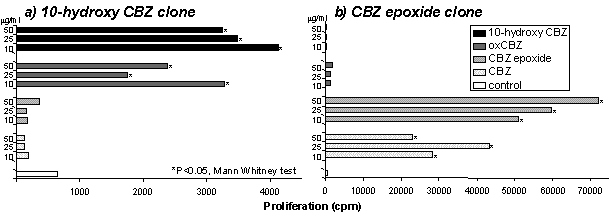
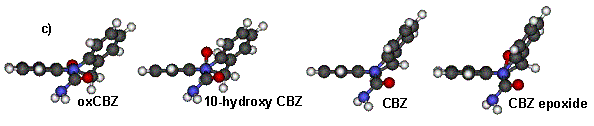
Fig 1. (a,b) T-cell proliferation (c) Energy minimized confirmations. These studies confirm that CBZ binds directly to MHC and the T-cell receptor. CBZ and CBZ metabolites activate T-cells. Since we have identified a relationship between the structure of the drug and T-cell activation, molecular modelling may assist future prediction of whether drug and T-cell receptor interactions will occur.
Naisbitt DJ, et
al. 2003a. Mol Pharmacol. 63: 732-41.
Naisbitt DJ, et al. 2003b. J Allergy Clin Immunol. 111:
1393-403.
These studies were funded by The Wellcome Trust.