| pA2 online © Copyright 2004 The British Pharmacological Society |
203P
GKT, University of London Winter Meeting December 2003 |
|
Characterization
of T-cell responses in phenindione hypersensitivity |
|
Phenindione is an oral anticoagulant with the same clinical indications as warfarin. 1.5-3% of all patients develop cutaneous manifestations, which may prevent effective therapy (Mcmenamin et al., 1976). Clinical symptomatology and the delayed onset of the reaction suggest an immune pathogenesis; however, to date antigen-specific T-cells have not been identified. The aims of this study were (1) to diagnose phenindione hypersensitivity using the lymphocyte transformation test and (2) to clone antigen-specific T-cells to characterise the cellular pathophysiology of the reaction.
Lymphocytes were
obtained from a 38-year old female who developed a maculopapular rash,
pneumonitis and eosinophilia following administration of phenindione and
for comparison, from 4 patients on phenindione without hypersensitivity.
Lymphocyte proliferation was measured by incubating cells (1.5x105;
total volume 0.2ml) with phenindione (1-500µg/ml) for 6 days (37oC;
5% CO2). [3H]
thymidine (0.5µCi) was added for the final 16h. T-cells were cloned
by serial dilution and repetitive mitogen stimulation and characterized
in terms of their CD and T-cell receptor phenotype. Proliferation and
cytokine secretion (IFN-![]() ,
IL-4, 5 and 10) were measured by [3H]-thymidine
incorporation and ELISA, respectively. The specificity of the drug T-cell
receptor interaction was studied using phenindione and 8 structurally
related compounds. To study whether processing is a prerequisite for phenindione
presentation, antigen presenting cells were fixed with glutaraldehyde.
Fixed antigen presenting cells cannot process drug antigens (Naisbitt
et al., 2003). Statistical analysis was performed by the Mann-Whitney
test.
,
IL-4, 5 and 10) were measured by [3H]-thymidine
incorporation and ELISA, respectively. The specificity of the drug T-cell
receptor interaction was studied using phenindione and 8 structurally
related compounds. To study whether processing is a prerequisite for phenindione
presentation, antigen presenting cells were fixed with glutaraldehyde.
Fixed antigen presenting cells cannot process drug antigens (Naisbitt
et al., 2003). Statistical analysis was performed by the Mann-Whitney
test.
Lymphocytes from
the hypersensitive patient proliferated on stimulation with phenindione
(figure 1a). No proliferation was observed from control patients. 43 drug-specific
T-cell clones were generated, 12 of which were selected for further analysis.
All 12 Clones were CD4+ and expressed the ![]() ß
T-cell receptor. Analysis of cytokines secreted from 8 phenindione stimulated
T-cell clones revealed a mixed cytokine secretion profile. Moderate to
high levels of IFN-
ß
T-cell receptor. Analysis of cytokines secreted from 8 phenindione stimulated
T-cell clones revealed a mixed cytokine secretion profile. Moderate to
high levels of IFN-![]() (9189±11001pg/ml; range 1024-23980), was secreted by five clones,
four clones secreted IL-5 (3459±4009pg/ml; range 642-9364), and
one clone secreted IL-4 (4914pg/ml). 8 clones were highly specific and
proliferated in the presence of phenindione alone; however, two clones
were stimulated by S-warfarin, 1 clone was stimulated by R- and S-warfarin
(figure 1b) and one clone was stimulated by 2-phenylindene. Phenindione
was presented to 7/10 clones by glutaraldehyde fixed antigen presenting
cells.
(9189±11001pg/ml; range 1024-23980), was secreted by five clones,
four clones secreted IL-5 (3459±4009pg/ml; range 642-9364), and
one clone secreted IL-4 (4914pg/ml). 8 clones were highly specific and
proliferated in the presence of phenindione alone; however, two clones
were stimulated by S-warfarin, 1 clone was stimulated by R- and S-warfarin
(figure 1b) and one clone was stimulated by 2-phenylindene. Phenindione
was presented to 7/10 clones by glutaraldehyde fixed antigen presenting
cells.
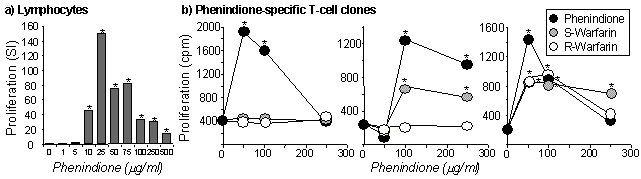
Figure 1. (a) Lymphocyte proliferation. (b) Cross-reactivity of T-cell clones. Co-efficient of variation less than 20% (*P<0.05).
In conclusion, these data show that phenindione hypersensitivity is orchestrated by CD4+ drug-specific T-cells. The interaction between phenindione, MHC and the T-cell receptor is highly specific (i.e., small structural changes inhibited T-cell activation); phenindione was presented via two pathways, one dependent and the other independent of antigen processing. Warfarin administration to phenindione hypersensitive patients may lead to the development of hypersensitivity but this needs to be evaluated in a clinical setting.
Mcmenamin RA, et
al. (1976) Aust N Z J Med 6:583-7.
Naisbitt DJ, et al. (2003) J Allergy Clin Immunol. 111:1393-403.
These studies were funded by The Wellcome Trust.
Pfizer Poster Communication Prize Winner.