| pA2 online © Copyright 2004 The British Pharmacological Society |
208P
GKT, University of London Winter Meeting December 2003 |
|
A comparison
of methods to predict drug clearance in neonates, infants and children |
|
Drug clearance (CL) values in paediatric patients can be predicted using allometric scaling factors such as body weight (BW), body surface area (BSA) and the body weight ¾ power model (ALL)1. However, below 1 year of age these 'scaling for size' methods over predict drug CL as they fail to account for the lack of fully developed renal and hepatic functions. Rates of development in these functions during the first year of life are much greater than can be accounted for by the rate of growth as assessed by measures of body size.
The SIMCYP software developed at the University of Sheffield to incorporate genetic, physiological, demographic and clinical variability into in vitro-in vivo extrapolation has previously been used to predict the CL of drugs in children down to 18 months of age2. Information on early physiological development and the ontogeny of specific cytochrome P450 enzymes, from birth onwards has now been incorporated into the software. Thus, the aim of this study was to predict the CL of oral caffeine and intravenous midazolam in children from birth onwards using the SIMCYP software and to compare the results with those from allometric methods.
Paediatric CL values were calculated by applying BW, BSA, and ALL adjustments to CL values from adults. These together with in vitro-in vivo derived values of CL in paediatrics (SIMCYP) were compared to in vivo values obtained from the literature. Mean squared prediction error (mse) and mean prediction error (me) were used as measures of precision and bias respectively. The results for caffeine and midazolam are shown in Figure 1. Different approaches ranked on their bias as: (a) under 2 years of age: BSA>ALL>BW>SIMCYP (b) over 2 years of age: BW>BSA>SIMCYP>ALL, Corresponding ranking with regard to precision were: SIMCYP>ALL>BSA>BW for all ages.
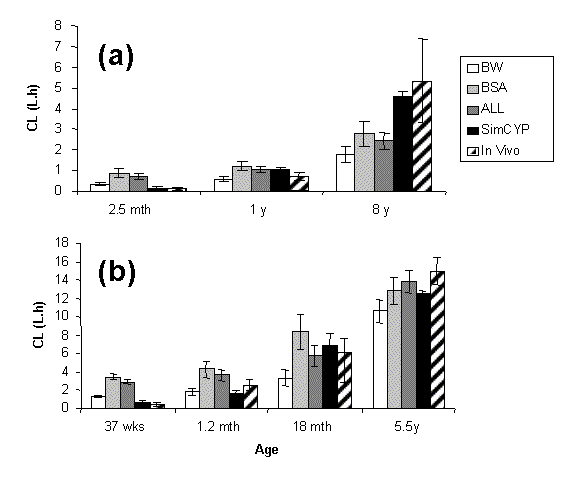
Figure 1. Comparing allometric (BW, BSA, ALL & LV) and more physiologically based (SIMCYP) methods for predicting oral clearance of caffeine (a) and i.v clearance of midazolam (b) in different paediatric groups.Application of more physiologically based predictions of drug clearance in paediatrics (SIMCYP) is shown to be superior to that of simplistic allometry (especially in <2 yrs). Although such in silico simulations cannot replace clinical studies they provide the best estimates to be used in the design of such studies.
1.Anderson et al
(2002) Paediatric Anaesthesia 12: 205 - 219.2.
2.Johnson et al (2003) Br J Clin Pharmacol 55 : 432.