| pA2 online © Copyright 2004 The British Pharmacological Society |
215P
GKT, University of London Winter Meeting December 2003 |
|
Investigation
of the effects of inhaled tiotropium on the response to acetylcholine
in the forearm vasculature |
|
Tiotropium is a recently introduced long-acting, inhaled, anti-muscarinic, bronchodilator drug, that is an effective treatment for chronic obstructive pulmonary disease when administered once daily (Hvizdos et al, 2002). Tiotropium has approximately equal affinity for each of the 5 known muscarinic receptor subtypes but dissociates slowly from the M1 and M3 subtypes, with dissociation half lives of 14 hours and 34.7 hours respectively. Its action is, therefore, relatively M1- and particularly M3-specific. Systemic anticholinergic effects of inhaled tiotropium have been reported from clinical trials. These include dry mouth, constipation, urinary difficulty and, uncommonly, tachycardia. However, the potential systemic vascular effects of local administration to the lung have not been investigated in detail. Acetylcholine (ACh) is a muscarinic agonist that causes vasodilatation in the forearm when infused at the brachial artery. It is not entirely clear which receptor subtypes mediate this effect, although indirect evidence suggests that the M3 subtype is important (Bruning et al, 1994).
The aim of the study was to investigate the systemic vascular effects of inhaled tiotropium by characterising its effect on the dose response curve of ACh-mediated vasodilatation in the forearm.
Eight healthy subjects (males) were studied in a two-way, randomised, placebo-controlled, single blind crossover study. On the 2 study days, after resting quietly for 10 minutes, the brachial artery of the non-dominant arm of each subject was cannulated with a 27-SWG cannula, under local anaesthesia (0.1% lignocaine), and subjects received a 30 minutes infusion of saline (1ml/min); during this time, baseline forearm blood flow (FBF) measurements were made, using venous occlusion plethysmography (Wilkinson & Webb, 2001). Subjects then received a 6-minute intra-arterial infusion of ACh (Clinalfa) at four different doses (2, 6, 20 and 60 µg/min), with FBF assessed during the last 3 minutes of each infused dose. At the end of the baseline ACh infusions, subjects received Tiotropium 18µg (as Spiriva) or placebo at 2 different time points and, after 1 hour from each dose, its effect assessed on ACh-mediated vasodilatation. Blood pressure and heart rate were recorded every 12 minutes throughout the study. Cardiac Index was also recorded every 30 minutes using a non-invasive transthoracic electrical bioimpedance technique.
No difference was detected in the response to different doses of ACh between tiotropium and placebo (Figure 1).
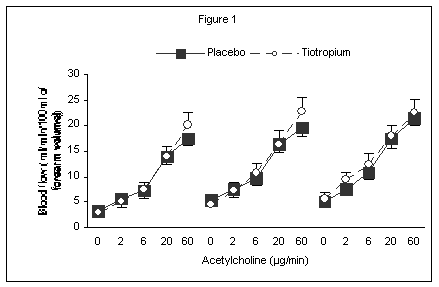
Tiotropium does not affect the response to ACh in the human forearm vasculature. This result may suggest that neither the M1 nor the M3 receptor subtype mediate cholinergic vasodilatation in the forearm. An alternative explanation is that such a result might have been due to low systemic exposure to tiotropium, although this is unlikely given the side effects associated with lower doses. A limitation of the present study is that tiotropium plasma levels were not assessed. Nevertheless, we can conclude that clinically used doses of tiotropium should not interfere with the interpretation of studies based on the assessment of endothelial function using venous occlusion plethysmography.
1.Hvizdos KM, Goa
KL. (2002) Tiotropium bromide. Drugs. 62:1195-203.
2.Bruning TA, Hendriks MG, Chang PC, Kuypers EA, van Zwieten PA (1994)
In vivo characterisation of vasodilating muscarinic-receptor subtypes
in humans. Circ Res. 74:912-9.
3.Wilkinson IB, Webb DJ. (2001) Venous occlusion plethysmography in cardiovascular
research: methodology and clinical applications. Br J Clin Pharmacol.
52:631-46.