| pA2 online © Copyright 2004 The British Pharmacological Society |
218P
GKT, University of London Winter Meeting December 2003 |
|
Drug-specific
proliferation of lymphocytes from patients hypersensitive to carbamazepine
and oxcarbazepine: cross-reactivity and possible clinical implications |
|
Administration of
carbamazepine (CBZ) is associated with rare, but serious allergic reactions
characterised by skin involvement, eosinophilia and systemic manifestations.
We have recently isolated cytotoxic IFN-![]() secreting CD4+ T-cells from hypersensitive individuals but not CBZ exposed
non-allergic controls (Naisbitt et al., 2003). The drug and T-cell interaction
was MHC-restricted and highly regulated (i.e., small structural changes
inhibited proliferation). CBZ was presented to T-cells in the apparent
absence of drug metabolism and antigen processing.
secreting CD4+ T-cells from hypersensitive individuals but not CBZ exposed
non-allergic controls (Naisbitt et al., 2003). The drug and T-cell interaction
was MHC-restricted and highly regulated (i.e., small structural changes
inhibited proliferation). CBZ was presented to T-cells in the apparent
absence of drug metabolism and antigen processing.
Oxcarbazepine (oxCBZ; a keto derivative of CBZ), a pro-drug for 10-hydroxy CBZ, has similar efficacy to CBZ. Based on clinical symptomology, only 1 in 4 of CBZ allergic patients administered oxCBZ develop an allergic reaction (Jenson et al., 1986). Zakrzewska et al. (1988) studied the potential for T-cells from CBZ hypersensitive patients to cross-react with oxCBZ. Lymphocytes responded in the presence of CBZ but not oxCBZ and the authors concluded that oxCBZ may be a suitable alternative to CBZ in allergic patients. The authors however, did not explore potential cross reactivity with 10-hydroxy CBZ, a metabolite of CBZ and the active component of oxCBZ (Myllynen et al., 1998).
Thus, the aim of these studies was to measure in vitro drug specific proliferation of lymphocytes from 4 CBZ allergic and 1 oxCBZ allergic patients and 5 healthy controls. Patients all developed rash together with systemic symptoms within 6 weeks of starting the drug, and the rash settled on dechallenge. Lymphocytes were incubated with CBZ, CBZ 10,11 epoxide (a metabolite of CBZ), dihydro CBZ, cis & trans dihydroxy CBZ, 10-hydroxy CBZ and oxCBZ (10-100µg/ml) for 6 days (37oC; 5% CO2). Proliferation was measured by the addition of [3H] thymidine (0.5µCi) for the final 16h. Results are expressed as mean±SD stimulation index (SI; cpm in drug-treated cultures/cpm in cultures containing solvent).
Statistical analysis was performed by the Mann-Whitney test.Lymphocytes from CBZ allergic patients proliferated in the presence of CBZ (SI 11.7±3.3, range 6.9-14.3; 10µg/ml; P<0.05), CBZ 10,11 epoxide (SI 7.5±1.7, range 5.7-9.7; 50 or 100µg/ml; P<0.05), dihydro CBZ (3.8±1.2, range 2.8-5.5; 50 or 100µg/ml; P<0.05), 10-hydroxy CBZ (3.4±0.9, range 2.5-4.7; 50 or 100µg/ml; P<0.05) and oxCBZ (2.4±1.4, range 0.6-4.0; 10µ/ml; P<0.05), but not cis or trans dihydroxy CBZ (SI<1.5). Figure 1A shows concentration dependent proliferation of lymphocytes from one patient. Cells from the patient allergic to oxCBZ also proliferated with CBZ, CBZ 10,11 epoxide, oxCBZ and 10-OH CBZ (Figure 1B). Control patient cells did not proliferate.
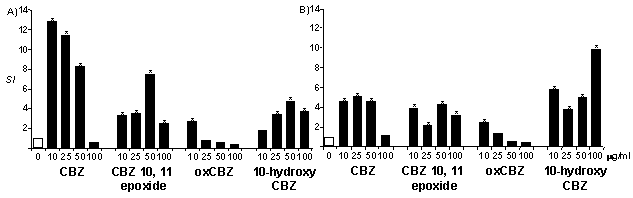
Figure 1. Proliferation of cells from (A) CBZ and (B) oxCBZ allergic patients. Co-efficient of variation less than 20%.These data, which differ from previous studies, identify lymphocytes from CBZ and oxCBZ allergic patients that proliferate with both CBZ and oxCBZ. This cross reactivity may be due to metabolism of both drugs to 10-hydroxy CBZ (Myllynen et al., 1998). We therefore recommend that administration of CBZ or oxCBZ to patients with well-characterised allergy should be carefully monitored.Jenson P, et al. 1986. Irish J Med Sci 155:297.
Myllynen P, et
al. 1998. Hum Exp Toxicol. 17:668-76.
Naisbitt DJ, et al. 2003. Mol Pharmacol. 63:732-41.
Zakrzewska JM, et al. 1988. J Allergy Clin Immunol. 82:110-5.
These studies were funded by The Wellcome Trust.