| pA2 online © Copyright 2004 The British Pharmacological Society |
226P
GKT, University of London Winter Meeting December 2003 |
|
Effect of
patient information leaflet on drug preference |
|
Doctors have long suspected that Patient Information Leaflets (PILs) enclosed with each drug pack influence the reporting of adverse effects (Lamb et al., 1994) although objective confirmation for this is lacking. The purpose of the current study was to investigate whether the PILs could potentially influence drug choice.
We asked 100 university students, excluding those studying medicine to rank their willingness to take 1 of 5 medicines for the treatment of an imagined diagnosis of hypertension. To guide this decision we provided modified PILs for a drug randomly selected from each of the five most commonly prescribed antihypertensive drug classes (Ca+2 channel blockers, ß-receptor antagonists, thiazide diuretics ACE inhibitors, and angiotensin II antagonists) along with a brief explanatory sheet and a questionnaire requesting rank for each drug, reason for rank given to 1st and last choice drugs and participants age and sex. PILs were modified only to remove drug names and information that would allow the drug class to be identified. The mean rank (1-5, 1 most favoured) allocated to each drug was analysed using Friedman's non-parametric ANOVA and using Dunn's test for post-hoc pairwise comparisons. The study complied with the then current guidelines for student research projects laid down by the South Sheffield Research Ethics Committee.
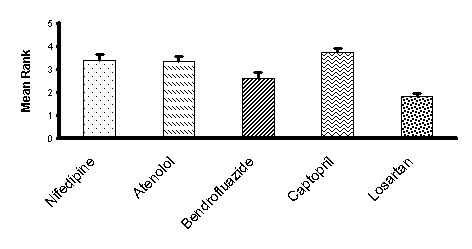
After 2 reminders 30 participants (11 male) returned questionnaires. The mean age of respondents was 19.1 years. There was a highly significant difference (p<0.0001) in mean rank allocated to each of the drugs (Fig 1). Losartan was significantly more highly favoured than all drug classes (p<0.005) other than bendrofluazide with no other significant pairwise differences. The most commonly cited reason for ranking drugs as most or least favoured was the number of adverse effects. Ease of use was also considered important.Although interpretation of the results of the current study is limited by the low response rate information contained in PILs clearly has the potential to influence attitude to antihypertensive drug treatment. This may either reflect the pharmacology of the drug or the mode of presentation in the PIL. There is clinical trial evidence of a difference in frequency adverse effects between angiotensin II antagonists and atenolol (Dahlöf et al., 2002) but it is of note that otherwise main drug classes appear equally well tolerated (Materson et al. 1993). These data make continued adherence in open studies (Caro et al., 1999) more difficult to interpret as the effect of drug and information in the PIL could be equally responsible for the apparent benefit of angiotensin II antagonists.
Caro JJ, (1999)et
al. CMAJ, 160:41-6.
Dahlöf B, et al.(2002) Lancet ,359: 995-1003.
Lamb GC, et al. Arch Int Med 1994, 154: 2753-6.
Materson BJ, et al. (1993 )N Engl J Med, 328:914-2.
Wood et al. (1993) N Engl J Med 19,328:914-21.