| pA2 online © Copyright 2004 The British Pharmacological Society |
229P
GKT, University of London Winter Meeting December 2003 |
|
Characterisation
of drug-specific CD4+ T-cells expressing the skin homing receptor
CCR10 in lamotrigine hypersensitivity |
|
Lamotrigine (LTG) is a widely used anticonvulsant with efficacy comparable to that of other antepileptic drugs. Unfortunately, LTG administration is associated with severe skin reactions that develop as part of a generalised hypersensitivity syndrome. We have recently identified drug-specific, CD4+ T-cells from patients with LTG hypersensitive patients, but not LTG-exposed controls (Naisbitt et al., 2003). Despite this, the reason why T-cells target skin is not understood.
Homey et al. (2002) and Vestergaard et al. (2003) have reported that skin infiltrating T-cells from patients with atopic dermatitis express the skin homing receptor cutaneous lymphocyte antigen (CLA) and chemokine receptors CCR4 and CCR10. It is possible that LTG-specific T-cells express homing receptors that facilitate transport and entry into skin. To address this issue, we have characterised the phenotype and cytokine and chemokine profiles of T-cell clones from a patient with LTG hypersensitivity and monitored skin homing and chemokine receptor expression.
A lymphocyte transformation test using cells from the hypersensitive patient was performed to ensure that the individual was sensitised against LTG. LTG-specific T-cells were then cloned by serial dilution, as described previously (Naisbitt et al., 2003), and characterized in terms of their CD and T-cell receptor phenotype by flow cytometry. To test the specificity of the clones, T-cells (0.5x105) were incubated with B-cells (0.1x105) and LTG (10 & 50µg/ml). After 48h, [3H] thymidine was added; results are expressed as mean±SD stimulation index (SI; cpm in drug-treated cultures/cpm in cultures containing solvent). Cytokine and chemokine profiles of LTG stimulated T-cell clones were monitored after 4h by RNase protection. Homing receptor (CD11a, CD18, CLA) and chemokine receptor expression (CCR3, 4, 6, 7, 10; CXCR3, 4, 6) was evaluated by flow cytometry.
Lymphocytes proliferated
vigourously with LTG (10µg/ml) (SI 9.6±1.4; Mann-Whitney
test, P<0.05). 9 CD4+/CD11a+/CD18+ T-cells clones were generated (SI
5.1±2.8 [10µg/ml], 5.9±3.9 [50µg/ml]; P<0.05).
Each clone expressed a single but differing T-cell receptor. Homing receptor
expression was measured on 5/9 T-cell clones. High levels of CCR10 and
low levels of CCR4 and CLA were expressed on all clones (Figure 1a). 2/5
T-cell clones expressed CXCR3 and CXCR4. LTG stimulation resulted in production
of the type 1 cytokine IFN-![]() and chemokines MIP-1
and chemokines MIP-1![]() and MIP-1ß; RANTES was expressed constitutively (Figure 1b). Up
regulation in TNF-
and MIP-1ß; RANTES was expressed constitutively (Figure 1b). Up
regulation in TNF-![]() ,
IL-4, IL-5, IL-8, IL-14 and I-309 mRNA expression was also observed with
certain LTG-stimulated clones.
,
IL-4, IL-5, IL-8, IL-14 and I-309 mRNA expression was also observed with
certain LTG-stimulated clones.
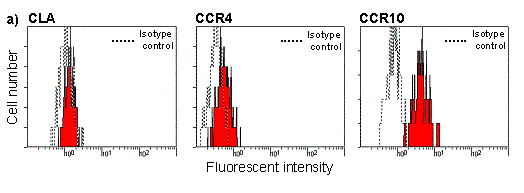

Figure 1. (a) Expression of skin homing receptors on LTG-specific T-cell clones. (b) Cytokine and chemokine mRNA from a LTG stimulated and unstimulated T-cell clone. These studies show that CD4+ LTG-specific T-cell clones preferentially express the skin homing receptor CCR10, which may facilitate T-cell migration to skin.
Homey B, et al.
Nat Med 2002; 8:157-65.
Naisbitt DJ, et al. J Allergy Clin Immunol 2003; 111:1393-403.
Vestergaard C, et al. Br J Dermatol. 2003; 149:457-63.
These studies were funded by The Wellcome Trust.