| pA2 online © Copyright 2004 The British Pharmacological Society |
020P
University of Buckingham 3th Focused Meeting April 2004 |
|
Orexigenic
peptide ghrelin in endothelial cells: vicinity to cardiovascular
receptor suggests paracrine mediator role |
|
Ghrelin, a 28 amino
acid peptide secreted mainly from stomach endocrine cells, is the endogenous
ligand of human GHS-R1a (Kojima et al., 1999) and known for its regulator
effects on growth hormone secretion and appetite. However, ghrelin receptors
have also been identified in human cardiovascular tissue and ghrelin is
a potent vasodilator both in vitro and in vitro(Katugampola et al., 2001;
Wiley & Davenport, 2002). These cardiovascular effects and the presence
of ghrelin mRNA in a wide range of peripheral tissues suggests the existence
of a site of ghrelin synthesis in cardiovascular tissues. Our aim was
to determine the cellular distribution of ghrelin and GHS-R1a in human
tissue using immunocytochemistry and fluorescent double staining.
Human tissue was obtained with ethical approval. Sections (30µm)
of fresh frozen ventricle (n=7 patients) atrium (n=5), coronary artery
(n=12), radial artery (n=6), left internal mammary artery (n=15), saphenous
vein (n=14), kidney (n=3) and lung (n=4) were stained using rabbit-anti-ghrelin
(RaG) or rabbit-anti-GHS-R1a (RaGR) primary antisera in a peroxidase-anti-peroxidase
protocol. Adjacent sections were double labelled using RaG antisera and
mouse-anti-van Willebrand Factor (vWF) monoclonal antibody (vWFma) or
RaGR antisera and vWFma or mouse-anti-smooth muscle a-actin monoclonal
antibody, detected by fluorescence labelled secondary antibodies. Sections
were viewed using a standard light or confocal laser scanning microscope.
Ghrelin-like immunoreactivity (ghrelin-LI) was detected in endothelial
cells of all vessels examined, confirmed by the co-localisation with the
endothelial marker vWF (Fig.1). GHS-R1a-like immunoreactivity (GHSR-LI)
was present in blood vessels and double staining with the endothelial
marker vWF or the smooth muscle marker a-actin revealed that GHSR-LI localised
to endothelial cells and vascular smooth muscle cells. GHSR-LI was also
present in atrial and ventricular cardiomyocytes.
Fig.1:Photomicro-graphs show ghrelin-like immunoreactivity in endothelial cells of small coronary arteries (a) and saphenous vein (b). Scalebar=50µm
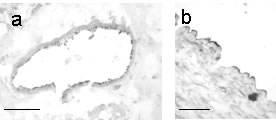
We report for the first time ghrelin-LI in vascu-lar endothelial cells of all tissues examined. This suggests that ghrelin is secreted from endothelial cells and may elicit cardiovascular effects acting on GHSR-LI, the receptor that we detected on cardiomyocytes and vascular smooth muscle cells. Furthermore, the presence of GHSR-LI on endothelial cells makes us hypothesise a negative feedback loop regulating endothelial ghrelin synthesis. Ghrelin may therefore represent a novel paracrine vascular mediator. In obesity, circulating ghrelin levels decrease (Shiiya et al., 2002). Obesity is also associated with atherosclerosis and endothelial dysfunction. We therefore speculate, that endothelial dysfunction in obese patients may result in impaired endothelial ghrelin synthesis. The resulting loss of ghrelin-mediated vasodilation may contribute to the development of hypertension in obese individuals.
Katugampola et al. (2001). Br J Pharmacol, 134, 143-9.
Kojima et al. (1999). Nature, 402, 656-60.
Shiiya et al. (2002). J Clin Endocrinol Metab, 87, 240-4.
Wiley et al. (2002). Br J Pharmacol, 136, 1146-52.