| pA2 online © Copyright 2004 The British Pharmacological Society |
044P
University of Bath Summer Meeting July 2004 |
|
Role of hydroxyl radicals in the mechanism of action of acetaminophen: comparison with inhibitors of COX-1 and COX-2 |
|
Acetaminophen (ApAP) has similar analgesic and antipyretic properties to nonsteroidal antiinflammatory drugs (NSAIDs), which act via inhibition of cyclooxygenase (COX) enzymes. However, unlike NSAIDs, ApAP is at best weakly antiinflammatory. COXs catalyze the conversion of arachidonic acid to prostaglandin H2, which serves as the common precursor for the synthesis of prostanoids (Mitchell & Warner 1999). Recent evidence indicates that the inhibitory effect of ApAP relies upon the reduction of the active oxidized form of COX to an inactive form (Ouellet & Percival 2001). Inhibition would therefore be more effective under conditions of low peroxide concentrations, consistent with the known tissue selectivity of ApAP (Boutaud et al. 2002). Here, we have examined the effects of peroxide on the inhibitory effects of ApAP and other NSAIDs on PGE2 production by A549 cells expressing COX-2.
A549 cells were incubated with IL-1ß (1 ng/ml) for 24 h to induce expression of COX-2 protein, which was not affected by ApAP (Figure 1A). For PGE2 production assays, the drugs were added to cells expressing COX-2 for 1h prior to addition of A23187 for 30 min. The inhibitory effect of ApAP on COX-2 activity was reduced by co-addition of t-butylOOH (EC50 580 µM; plus t-butylOOH > 3mM) (Figure 1B). In addition to ApAP, t-butylOOH also significantly (two way ANOVA) reduced the potency of the following NSAIDs, naproxen (EC50 12 mM; plus t-butylOOH 710 mM), ibuprofen (EC50 23 µM; plus t-butylOOH 320 µM) and rofecoxib (EC50 2.7 nM; plus t-butylOOH 35 nM). By contrast, t-butylOOH had no effect on the inhibition produced by diclofenac (EC50 5.6 nM; plus t-butylOOH 12 nM) or indomethacin (EC50 9.8 nM; plus t-butylOOH 14 nM).
(A)
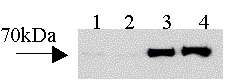
(B)
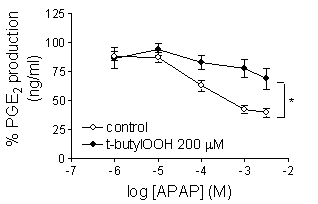
Figure 1. A; Effects of vehicle (1), vehicle plus ApAP (2), Il-1 ß (3) or IL-1 ß plus ApAP for 24 h on COX-2 expression in A549 cells. B; Effect of ApAP on COX-2 activity in the presence and absence of t-butylOOH (200 µM). Points indicate mean ± SEM, n=6-8.
Our data shows that an environment low in peroxides is required for ApAP to be an effective COX inhibitor.
Furthermore, we show for the first time that peroxide levels modulate the effects of a range of NSAIDs. Thus, NSAIDs (and ApAP) fit clearly into one of three categories; (i) those which are fully reversed, (ii) partially reversed or (iii) unaffected by organic peroxide levels. This information is important when considering the ‘actual’ selectivity of NSAIDs for different forms of COX.
Boutaud et al., (2002) Proc. Natl. Acad. Sci ., USA 99: 7130-7135
Mitchell and Warner (1999) Br. J. Pharmacol., 128: 1121-1132
Ouellet and Percival (2001) Arch. Biochem. Biophys., 387: 273-280
This work was supported by a Fellowship grant from the Spanish Government and grants from the Medical Research Council, the Joint Research Board of St. Bartholomew’s Hospital, and The William Harvey Research Foundation.