| pA2 online © Copyright 2004 The British Pharmacological Society |
052P
University of Bath Summer Meeting July 2004 |
Influence of β2-adrenoceptor polymorphisms on the inhibitory effects of isoprenaline on human lung mast cells L.J. Kay, L.K. Chong and P.T. Peachell. Academic Unit of Clinical Pharmacology, University of Sheffield, RHH (Floor L), Glossop Road, Sheffield, S10 2JF |
|
The β2-adrenoceptor (β2-AR) is polymorphic at a number of loci both within the promoter region and the coding block. These polymorphisms may influence receptor function (Leineweber et al., 2004). The aim of the present study was to establish whether these polymorphisms influence the ability of the β-AR agonist, isoprenaline (ISO), to inhibit histamine release from human lung mast cells (HLMC).
Human lung tissue was obtained from surgical resections. For genotypic analysis, genomic DNA was extracted from a small amount of lung tissue, the DNA amplified and genotype determined by Restriction Fragment Length Polymorphism or automated sequencing (Anyacioglu et al., 1999; Chong et al., 2000; Drysdale et al., 2000). The remainder of the lung tissue was physically and enzymatically disrupted to generate mast cell suspensions for use in histamine release experiments. Cells were incubated with or without ISO (0.1 nM – 10 µM) before challenge with anti-human IgE (1/300) for 25 min. Histamine release was determined by an automated fluorometric technique. Statistical treatments involved the use of Mann-Whitney and Kruskal-Wallis tests.
76 preparations were genotyped at 11 previously reported polymorphic positions. In this cohort, nucleotide positions -709, -406 and 100 were not polymorphic and, therefore, only the remaining 8 nucleotide positions (Table 1) were included for further analysis.
Table 1.
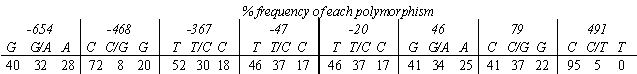
Potential correlations between these polymorphisms and the inhibitory effects of ISO on IgE-mediated histamine release from HLMC were investigated. For the 76 preparations, ISO inhibited histamine release in a concentration-dependent manner and maximal responses (Emax) and potencies (pD2) were determined. None of the polymorphisms influenced the ability of ISO to inhibit histamine release except that at nucleotide position 491. The polymorphism at this position causes an amino acid change at position 164 (thrile). However, this polymorphism is uncommon, with 72 preparations being homozygous (thr164thr) and 4 heterozygous 164(thr![]() ile). Comparison of these 2 groups indicates that ISO was significantly (P=0.002) more potent in homozygous than heterozygous preparations (pD2 values as mean ±s.e.m: 8.7 ±0.1 and 7.5 ±0.1, respectively).
ile). Comparison of these 2 groups indicates that ISO was significantly (P=0.002) more potent in homozygous than heterozygous preparations (pD2 values as mean ±s.e.m: 8.7 ±0.1 and 7.5 ±0.1, respectively).
These findings indicate that, of the 11 polymorphic positions of the β2-AR studied, only the thr164ile polymorphism may influence the inhibitory effects of ISO on HLMC.
Aynacioglu et al., (1999). Br. J. Clin. Pharmacol., 48, 761-764
Chong et al., (2000). Pharmacogenetics, 10, 153-162
Drysdale et al., (2000). PNAS, 97, 10483-10488
Leineweber et al., (2004). Naunyn-Schmiedeberg’s Arch Pharmacol., 369, 1-22