| pA2 online © Copyright 2004 The British Pharmacological Society |
068P
University of Bath Summer Meeting July 2004 |
|
Quantitative analysis of the internalisation of the CCR5 receptor using a high throughput confocal plate reader James Longden1,2, Emma-Louise Cooke1, Andy C Hargreaves1 and Stephen J Hill2, 1AstraZeneca R&D Charnwood, Loughborough, Leicestershire, LE11 5RH 2Institute of Cell Signalling, Queen’s Medical Centre, Nottingham, NG7 2UH |
|
Chemokine receptors are members of the G-Protein Coupled Receptor family of molecules that are found on white blood cells where they can direct the cells to sites of infection and inflammation. It has previously been suggested that the CCR5 receptor undergoes ligand induced internalisation into the perinuclear region of the cell via a clathrin-dependant pathway (Signoret et. al., 2000). In this communication a confocal imaging plate reader has been used to quantify this process.
CHO cells, stably transfected with the CCR5 receptor (tagged on the C-terminus with a Green Fluorescent Protein; GFP), were plated into 96-well Packard ViewPlates two days prior to experimentation (10,000 cells/well in 100 µl of media). Cells were serum starved for 24h, then treated with agonist for 5-90 min before being fixed with 2% paraformaldehyde and stained with the nuclear dye Hoechst 33342 (1 µM). Fluorescence of Hoechst 33342 (364nm excitation, 450nm emission) and GFP (488nm excitation, 535nm emission) was monitored using an IN Cell Analyser (Amersham; image size 0.745mm2 per well).
Trafficking of CCR5 to the perinuclear region was quantified using a granularity analysis algorithm, which measures distinct intracellular focal points (or grains) with pronounced fluorescence intensity differences from surrounding regions of the cell. Using this approach, MIP-1![]() , MIP-1ß and RANTES (EC50 = 0.94 ± 0.34nM, 6.65 ± 0.56 nM and 3.18 ± 0.48 nM respectively; mean ± s.e.mean; n=10; assayed 60 minutes after the addition of agonist) were shown to stimulate a concentration and time dependent internalisation of CCR5 (figure 1).
, MIP-1ß and RANTES (EC50 = 0.94 ± 0.34nM, 6.65 ± 0.56 nM and 3.18 ± 0.48 nM respectively; mean ± s.e.mean; n=10; assayed 60 minutes after the addition of agonist) were shown to stimulate a concentration and time dependent internalisation of CCR5 (figure 1).
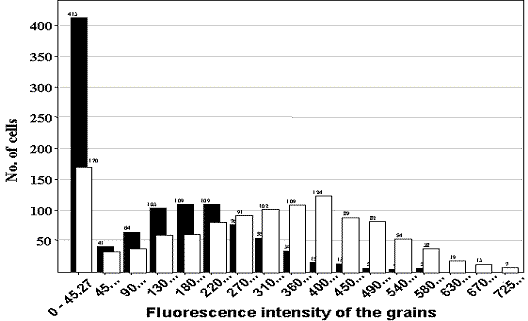
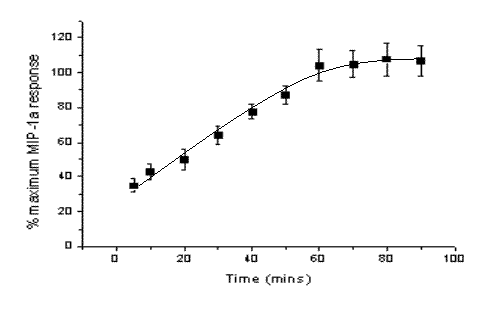
Figure 1. Individual cell data from one well of unstimulated cells are shown by the closed bars and corresponding data from cells stimulated for 60 minutes with MIP-1![]() (100nm) by the open bars, illustrating a Gaussian distribution of Fgrain (fluorescence of the grain) values and the shift in fluorescence intensity due to receptor internalisation. The change in receptor internalisation at different times after ligand (100nM MIP-1
(100nm) by the open bars, illustrating a Gaussian distribution of Fgrain (fluorescence of the grain) values and the shift in fluorescence intensity due to receptor internalisation. The change in receptor internalisation at different times after ligand (100nM MIP-1![]() ) addition is shown on the right. Fgrain values per cell have been averaged to generate a well value (n=5 separate experiments, 4 wells per condition).
) addition is shown on the right. Fgrain values per cell have been averaged to generate a well value (n=5 separate experiments, 4 wells per condition).
In summary, these experiments illustrate the generation of quantitative data from confocal images of randomly selected populations of cells (~1000 cells/well).
Signoret, N. et. al. (2000) Cell Biol. 151, (1281-1293)