| pA2 online © Copyright 2004 The British Pharmacological Society |
082P
University of Bath Summer Meeting July 2004 |
|
C-type natriuretic peptide inhibits angiotensin II-induced ERK 1/2 phosphorylation in rat aortic smooth muscle cells Yohann Rautureau, Caroline P.D. Wheeler-Jones, Gary F. Baxter. Veterinary Basic Science, Royal Veterinary College, London NW1 OTU |
|
Angiotensin II (Ang II)-induced proliferation of vascular smooth muscle cells is mediated by phosphorylation of extracellularly regulated kinase type 1 and 2 (ERK 1/2; Duff et al., 1992). C-type natriuretic peptide (CNP) is a vascular autocrine-paracrine factor produced mainly by endothelial cells (Kuhn, 2004). CNP inhibits proliferation of cultured rat aortic smooth muscle cells (RASMC) (Hutchinson et al., 1997). We hypothesised that CNP inhibits Ang II-induced ERK 1/2 phosphorylation in RASMC.
Male Sprague-Dawley rats (250-350 g) were killed by cervical dislocation. RASMC were isolated by explantation and used at passage 14 to 18. Following serum starvation overnight, cells were pre-incubated with human CNP for 30 min before stimulation with Ang II 100 nM for 30 min. ERK 1/2 phosphorylation was studied by immunoblotting using phospho-ERK 1/2 (Thr202/Tyr204) antibody and quantified by densitometric analysis. Data are expressed as mean ± s.e.m. Mean blot densities were compared using one-way analysis of variance.
In response to Ang II 100 nM, ERK 1/2 phosphorylation was increased 3-fold compared to control (P < 0.05) in RASMC. CNP alone did not modify basal ERK1/2 phosphorylation significantly. However, Ang II-induced ERK1/2 phosphorylation was attenuated significantly by CNP 1 pM, 10 nM or 1 µM (P < 0.05 vs Ang II alone). The inhibitory effect of CNP was maximal at 1 µM (35% relative decrease in Ang II-induced ERK1/2 phosphorylation) and was near maximal at 1 pM (32% relative decrease).
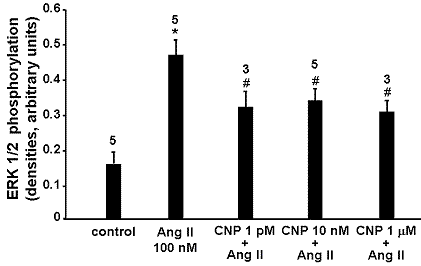
Figure 1: CNP decreases Ang II induced ERK 1/2 phosphorylation in RASMC. Numbers of experiments are indicated at the top of each bar. *, P< 0.05 vs control; #, P< 0.05 vs Ang II alone
These data indicate that CNP attenuates Ang II-induced ERK1/2 phosphorylation in RASMC, even at low physiological concentrations. This observation suggests that the proliferative effects of Ang II in vascular smooth muscle cells could be inhibited by CNP.
Duff et al., (1992). Biochem. Biophys. Res. Commun.188, 257-264
Hutchinson et al., (1997). Cardiovasc. Res., 35, 158-167
Kuhn (2004). Basic Res. Cardiol., 99, 76-82