| pA2 online © Copyright 2004 The British Pharmacological Society |
115P
University of Bath Summer Meeting July 2004 |
|
Phenotypic characterization of ETB deficient mice reveals ETA receptors are downregulated A.P. Davenport & R.E. Kuc, Clinical Pharmacology Unit, University of Cambridge, Level 6, Centre for Clinical Investigation, Box 110, Addenbrooke’s Hospital, Cambridge. CB2 2QQ. U.K |
|
We have previously shown in a rat model of hypertension, where adenovirus transfer of the prepro-ET-1 gene increased plasma ET-1 elevating systemic blood pressure, there was a significant compensatory downregulation in ETA receptor density correlating with reduced function in peripheral vessels but no change in ETB receptors (Télémaque-Potts et al2002). Our aim was to determine the relationship between ETB and ETA receptors (Davenport 2002) by comparing the density of each subtype in ETB knockout mice, where plasma ET levels are also elevated.
Following euthanasia, sections (15µm) were cut from the brains of 6 male (+/+) control, aged 29±3 days and 7 male ETB receptor knockout mice (-/-), aged 25±2 days (Hay et al 2001). Competition binding assays were carried out (Davenport et al 2002) using a fixed concentration of [125I]-ET-1 (0.1 nM,) and increasing concentrations of BQ-3020 (20 pM-100 µM) for one hour. One µM ET-1 defined the non-specific binding (NSB). Binding parameters were calculated using non-linear iterative curve fitting programmes (KELL, Biosoft, Cambridge, U.K.) and compared using Student's t-test. To further confirm the absence of ETB receptor expression, longitudinal adjacent sections of the remaining whole torso, cut from the midline were incubated with either 0.25 nM [125I]-BQ3020 or with 0.25 nM [125I]-PD151242, to visualise the ETB and ETA sub-types respectively. The autoradiographical distribution of receptor sub-types was also compared by incubating [125I]-ET-1 (0.1 nM) with 0.1 µM BQ123 to block ETA binding and visualise ETB receptors. ETA receptors were visualised by incubating adjacent sections with 0.1 µM BQ3020. In autoradiographical images of whole body sections of control mice, [125I]-BQ3020 binding localised to ETB receptor-rich organs in peripheral tissues including kidney and brain as expected but could not be detected in these tissues in ETB deficient animals, confirming gene deletion.
In control brains, unlabelled BQ3020 competed for [125I]-ET-1 biphasically (Table 1) with a more abundant high affinity site corresponding to the ETB receptors and a low affinity, micromolar ETA site, giving a selectivity for BQ3020 of over 200 fold. In the knockout mice however, BQ3020 did not compete for the high affinity ETB site in agreement with the autoradiographical data. There was no change in the affinity for the ETA receptor compared with control but importantly ETA receptor density was significantly reduced by 45%.
Table 1
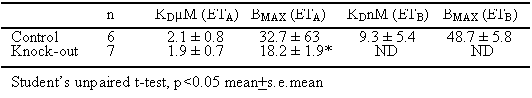
These results reveal deletion of the ETB sub-type is associated with a reduction in both central and peripheral ETA number and may reflect an unsuspected role for ETB receptors in regulating development or expression of the ETA sub-type. The density of the ETA receptors was downregulated by nearly half in the brain. Since ET-1 does not cross the blood-brain barrier, these results suggest an unexpected modulation of ETA receptors by ETB in the CNS.
Davenport, A.P. (2002). Pharmacol Rev;54:219-26
Davenport A.P., Kuc, R.E. (2002). Methods Mol Biol; 206:45-70
Hay, D.W., et al. ( 2001). Br J Pharmacol; 132:1905-15
Télémaque-Potts S., et al. (2002). Clin Sci;103 Suppl 48:357S-62S