| pA2 online © Copyright 2004 The British Pharmacological Society |
101P
University of Newcastle Winter Meeting December 2004 |
A refinement of the Ghosh and Schild in vivo anaesthetised rat lumen-perfused stomach gastric acid assay Elliot Lilley1, Nigel Shankley2, Sonia Roberts1, Mark Shaxted1, Robert Hull1. 1James Black Foundation, Dulwich, London. UK. 2Johnson & Johnson PRD, San Diego, California, USA. |
|
We have developed a modification of the traditional anaesthetised rat lumen perfused stomach technique first described by Ghosh and Schild (1959) which permits perfusion of the stomach without the need for abdominal surgery and which allows intragastric (i.g.) drug administration. Male Wistar rats (250-350g) fasted overnight were anaesthetised with urethane. All animals received a tracheotomy and both jugular veins were cannulated. The oesophagus was cannulated at the throat and a dual lumen, central venous paediatric catheter passed into the stomach. The inputs of the catheter were connected via three-way taps to a peristaltic pump in opposing directions to ensure that ‘in-flow’ and ‘out-flow’ were balanced. To initiate and facilitate stomach perfusion a 4ml volume of mucosal solution (mM: NaCl 118, KCl 4.8, CaCl2 0.65, glucose 31.6; pH 6.5; 37°C) was administered into the stomach. The stomach was perfused with this solution at a rate of 1ml min-1. Upon leaving the stomach, the perfusate was passed though a glass flow cell into which a pH electrode was placed for continuous measurement of pH (see figure 1). Each animal received an intravenous (i.v.) infusion of pentagastrin at a rate of 0.1µg kg-1 min-1 (an approximate half maximal acid secretory dose) for the entire duration of the experiment. i.v. bolus doses of test compound were given in a 200µl volume, followed by 400µl saline. i.g. dosing was performed by stopping the perfusion pump and administering 2ml of test compound into the stomach. The perfusion was re-started 10min after drug administration and responses monitored.
Results from two example gastrin receptor antagonists JB95008 and YM220, non-orally active and orally active respectively are given in figure 2.
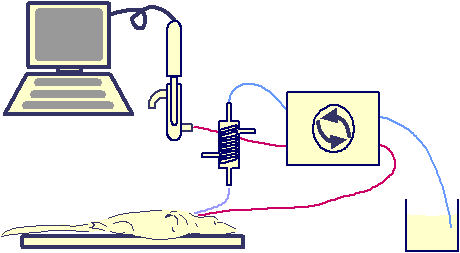
Figure 1
The experimental set-up. The aneasthetised rat (d) has musosal solution passed from a reservoir (a) into its stomach via heating coil (c) by a peristaltic pump (b). The action of the pump also allows for the fluid within the stomach to pass out and into a glass flow cell (e) containing a pH electrode (f). The pH of the fluid in contact with the electrode is monitored by PC using data acquisition software.
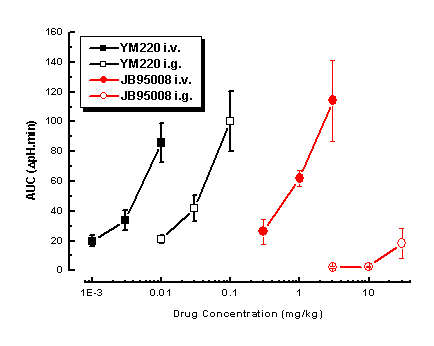
Figure 2:
Graph showing the dose-response relationships for JB95008 (circles) and YM220 (squares) following i.v. (closed symbols) and i.g. (open symbols) administration on pentagastrin-stimulated gastric acid secretion in anaesthetised rats (all symbols represent the mean and standard error from 4-6 replicate experiments).
These data are consistent with a great many other experiments conducted using this assay and illustrates that this approach provides a reliable indication of oral activity of test compounds in the anaesthetised rat.
This assay represents a refinement of the assay first described by Ghosh and Schild and will result in a reduction on the total number of animals used to characterise potential anti-gastric acid secretory molecules.
Ghosh & Schild (1958) Br. J. Pharmacol 13, 54-61.