Print version
Search Pub Med
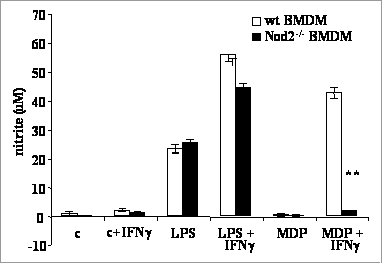
Interferon-γ enhances production of nitric oxide from macrophages stimulated by pathogen associated molecular patterns via a mechanism that depends on Nod-2 Pattern recognition receptors (PRRs) are central to the induction of innate and acquired immunity via their roles in the recognition of pathogen associated molecular patterns. Important classes of PRRs include the Toll-like receptors (TLR) and the Nucleotide oligomerisation domain (Nod) receptors (Beutler et al., 2003, Philpott and Girardin, 2004). Mice lacking the pattern recognition receptor, TLR4, respond very poorly to stimulation by lipopolysaccharide (LPS), but administration of the cytokine interferon gamma (IFN-γ has been described as restoring apparent sensitivity to this stimulatory ligand by an unknown mechanism. Primary bone marrow macrophages (BMMs) were isolated from the femur and tibia of mice killed by cervical dislocation and cultured as described (Royle et al., 2003). Uridine 5’diphosphoryl N-acetyl (UDPmurNac)-L-alanyl-D-glutamate and UDPmurNac-L-alanyl-D-glutamyl-meso 2,6-diaminopimelate were synthesised from UDP-N-acetyl glucosamine. Nitric oxide (NO) levels in the supernatant of the cultured cells were determined using the Griess assay. Tumor necrosis factor (TNF)-γ was detected by an ELISA (R&D systems, UK). Figure 1: No production from BMM (wild type (WT) or Nod2-/-) in response to MDP
IFN-γ -primed TLR4-/- BMM stimulated with un-purified LPS produced NO, but not TNF-γ . IFN-γ -primed TLR4-/- BMM stimulated with highly purified LPS did not produce NO. Production of TNF-γ was low in response to all of the peptidoglycan intermediates whether or not the cells were primed with IFN-γ . Administration of muramyl-N-acetyl di- (MDP) and tri-peptides to the cells (in lipofectamine transfection reagent or in solution) induced NO production from IFN-γ primed BMM, whereas peptide mixtures of the constituents for the di- and tri-peptides had no effect on these cells. NO production was independent of TLR4, TLR2, MyD88 and CD11b whereas IFN-γ -primed NO production was lost in BMM from Nod-2-/- mice (figure 1). This study shows that IFN-γ primed macrophages produce NO in response to MDP through Nod-2. The effect of IFN-γ in restoring inflammatory responses to Gram negative bacteria or bacterial products in mice with defective TLR4 signalling is likely to be due, at least in part, to a response to peptidoglycan, not LPS. Beutler, B., et al., (2003) J Leukoc Biol 74, 479-485. |