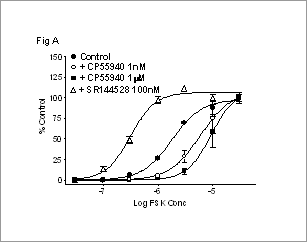
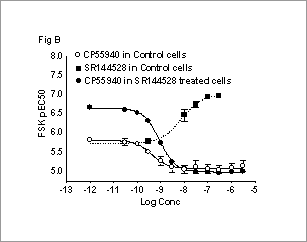
Characterisation of CB2 agonist effects on forskolin-stimulated changes in cellular cyclic AMP 7TM receptors that couple through the Gi family of G-proteins are known to inhibit adenylyl cyclase (AC) and inhibit forskolin (FSK) stimulated increases in cellular cAMP levels. In this study we have studied in detail the effect of activating the Gi-coupled cannabinoid CB2 receptor on FSK-stimulated increases in cellular cAMP. cAMP was measured indirectly using a reporter gene assay (Bouaboula et al., 1997). Studies were performed using CHO-K1 cells expressing human recombinant CB2 receptors and a recombinant Luciferase gene under transcriptional control of a cAMP response element (CRE-LUC). Cells were harvested using Versene and suspended in serum-free DMEM/F12 assay buffer. FSK and CB2 ligands were incubated with cells for 4-5hr at 37oC. cAMP-dependent production of Luciferase was measured using Luclite reagent (Amersham Biosciences). Data are the mean +SEM of 3 experiments. In some studies, cells were pre-treated with 100nM SR144528 (Bouaboula et al., 1999) for 30min, and then extensively washed, before harvesting. FSK stimulated Luciferase production in a dose-dependent manner (Fig A). The CB2 agonist, CP55940, decreased the FSK pEC50 while the inverse agonist, SR144528, increased the FSK pEC50 (Fig B). Treatment of cells with SR144528 increased FSK pEC50 (vs control cells). CP55940 reduced the FSK pEC50 in these cells (Fig B).
A) Effect of CP55940 and SR144528 on FSK responses in control cells.
B). Effect of CP55940 and SR144528 on FSK pEC50 in control and SR144528 treated cells. These data suggest that inhibition of FSK-stimulated cAMP accumulation by CB2 receptors is due to a saturable decrease in FSK pEC50. The effect of SR144528 on FSK responses suggests that CB2 receptors are constitutively active in our cell line and the increase in FSK pEC50 produced by SR144528 is consistent with CB2 receptor activation modulating FSK potency. The mechanism by which FSK potency is modulated by CB2 agonists is not known but may be due to Gi-subunits liberated by CB2-receptor activation affecting the FSK-AC interaction to either decrease the affinity of FSK for binding to AC or to decrease the catalytic activity of AC.
Bouaboula, M., et al . (1997). J. Biol. Chem., 272, 22330-22339. |
|