Role of the C-terminus in ghrelin receptor internalisation The appetite stimulating actions of the stomach hormone ghrelin are mediated by a constitutively active, Gq/11 protein coupled ghrelin receptor (GhR), that undergoes rapid basal internalisation and recycling (Holst et al., 2003; Holst et al., 2004a,b). In contrast GPR39 is a related orphan receptor with similar basal activity, but which does not undergo endocytosis (Holst et al., 2004b). The receptor C terminus plays a crucial role in recruitment of β-arrestin ( βarr) clathrin adaptors, and here we investigate its importance in GhR endocytosis using chimeric GhR and GPR39 proteins. C termini were exchanged following the NPXXY domain (TM VII), to generate FLAG-GhR with GPR39 C terminus (GhR-39ct) and vice versa (39-GhRct). Transfected HEK293 cells (Lipofectamine 2000) were surface labelled with M2 anti FLAG antibody and vehicle, 100 nM ghrelin (5 or 15 min) or 100 μM ZnCl2 (a GPR39 agonist) added, including transferrin (Tf)-Texas Red. Immunofluorescent detection (AlexaFluor488 or 594) and quantitative analysis of internalisation and Tf-colocalisation (Volocity 3.1; Improvision) was performed as described before (Holst et al., 2004b). Results are presented from three independent experiments, in each case calculating mean internalisation and Tf-colocalisation for four representative cells. Significance between data groups was assessed by one way ANOVA and Student-Newman-Keuls post test.
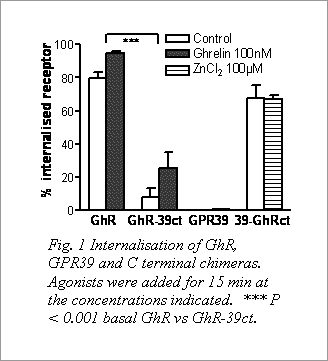
Fig. 1 Internalisation of GhR, GPR39 and C terminal chimeras. Agonists were added for 15 min at the concentrations indicated. *** P < 0.001 basal GhR vs GhR-39ct. Unstimulated GhRs were intracellularly distributed, with further internalisation after ghrelin (Fig.1) to punctate compartments colocalised with Tf (72.4±7.6 %, n = 3). The exclusive plasma membrane distribution of GPR39 was unaffected by Zn2+. In contrast GhR-39ct was mainly cell surface localised under basal conditions, and underwent further endocytosis following ghrelin (Fig. 1; 31.4±7.8 % overlap with Tf, n = 3). 39-GhRct immunoreactivity was rarely detected by live labelling, but was also intracellularly distributed, with low Tf-colocalisation (20.9±2.5 %, n = 3 compared to 68.9±6.3 % for GhR control, n = 3; P < 0.001). GFP-βarr2 recruitment to endosomes occupied by the GhR was observed principally after ghrelin (5 min), and was reduced for the GhR-39ct chimera. GPR39 did not associate with GFP- βarr2 under basal or Zn2+-stimulated conditions. We conclude that the unique trafficking profiles of GhR and GPR39 are defined by the C terminal domains of these receptors, and that the GhR C terminal domain is responsible for βarr2 endosomal recruitment after ghrelin stimulation.
Holst, B. et al. (2003) Mol. Endocrinol. 17 , 2201-2210. |