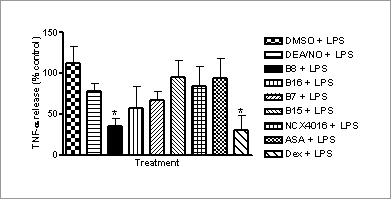
A novel hybrid aspirin-nitric oxide donor drug inhibits TNF-α release from LPS-activated human macrophages in vitro Non-steroidal anti-inflammatory drugs (NSAIDs) modulate inflammation primarily through inhibition of cyclo-oxygenase-mediated synthesis of pro-inflammatory prostanoids (Dannhardt & Kiefer, 2001) . A common side-effect of NSAID administration is the development of gastric ulcers; hybrids of aspirin with nitric oxide (NO) donor moieties have shown some benefit in avoiding gastric injury (Cena et al., 2003) , but their anti-inflammatory effects have not been fully explored. Here, we set out to determine the anti-inflammatory properties of novel NO-releasing furoxan derivatives of aspirin in activated human monocyte-derived macrophages. Peripheral venous blood was drawn from the antecubital fossa of human volunteers (non-smokers; age 20-45). Mononuclear cells were isolated from human blood using dextran sedimentation/Percoll gradients and resuspended at a concentration of 4x106 ml -1 in ISCOVES Dulbecco’s modified Eagle’s Medium (DMEM). Macrophages were derived from the monocytes (enriched by adherence) by culturing them in DMEM supplemented with 10% autologous serum (37 °C, 7 days). On day 7, the medium in each well was changed to that containing 10 μM of either a furoxan aspirin; (3-cyanofuroxan-4-yl)methyl 2-acetoxybenzoate (B8) or (3-carbamoylfuroxan-4-yl)methyl 2-acetoxybenzoate (B7), their respective furazan NO-free counterparts; (4-cyanofurazan-3-yl)methyl 2-acetoxybenzoate (B16) or (4-carbamoylfurazan-3-yl)methyl 2-acetoxybenzoate (B15), aspirin, an existing nitroaspirin (NCX4016), a spontaneous NO donor, diethylamine diazeniumdiolate DEA/NO or dexamethasone (1 μM), with and without lipopolysaccharide (LPS;10 ng.ml-1). Cells were then incubated at 37 °C for 4 h and human TNF-α and IL-8 ELISA assays were conducted on the supernatants removed from macrophage plates. B8 had a significant inhibitory effect on TNF-α release in human monocyte-derived macrophages treated with LPS (Figure 1; p<0.05, 1-Way ANOVA followed by Dunnett’s test; n=8-10) but not in macrophages without LPS. The effect was equivalent in magnitude to that of dexamethasone, but was not shared by DEA/NO, B7, the furazans, aspirin or NCX4016. None of the drugs studied affected IL-8 release (p>0.05) or induced cell death, as assessed by lactate dehydrogenase assay (p>0.05; n=8-10).
Fig.1. Effect of potential anti-inflammatory agents on LPS-induced TNF-α release in human monocyte-derived macrophages. The lack of effects by B16 and aspirin, suggest that the inhibitory effect of B8 on TNF- α release in human monocyte-derived macrophages is NO-mediated. However, as this effect is not mimicked by the NO donor, DEA/NO, it is apparently a specific property of B8 that requires further exploration.
Cena C, et al., (2003) J Med Chem 46, 747-54. |