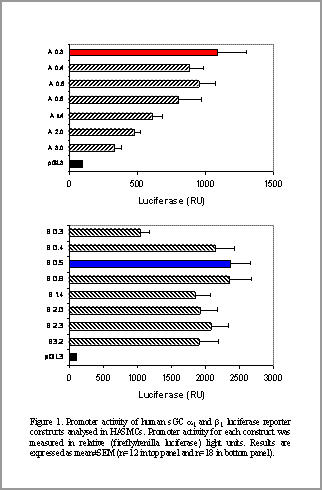
Characterisation of α1 and ß1 soluble guanylate cyclase promoter activity As a principal intracellular receptor for nitric oxide (NO), soluble guanylate cyclase (sGC) plays a fundamental role in cardiovascular homeostasis and disease. However, there is a paucity of information regarding expressional regulation of sGC in the vasculature. Since the enzyme is an obligatory heterodimer composed of α and β subunits (Kamisaki et al., 1986), we investigated the transcriptional regulation of human sGC α and β genes in primary human aortic smooth muscle (HASMCs; Promocell) and COS-7 cells (ATCC). The 5’ flanking regions harbouring 0.3-3.0 kb of both human α and β sGC genes were isolated and analysed for promoter activity by using luciferase-reporter constructs.
P <0.05) are activators of β1 sGC transcription in COS-7 cells but not in HASMCs, suggesting potential cell- or tissue- specific regulation. Upstream fragments of 0.3 kb and 0.5 kb exhibited maximal promoter activity for the α 1 and β sGC promoters, respectively (Figure 1). MatInspector software was used to identify putative transcription factor (TF) binding sites in the 5 ′ flanking region of both α and β sGC promoters proximal to their transcriptional start site. The functional significance of consensus TF binding sites was investigated by site-specific deletions in both 0.3 kb (α sGC) and 0.5 kb (β sGC) promoter fragments. Our data reveal that CBF (2.5±0.3-fold, n=3, P<0.05) and NF-κB (1.8±0.1-fold, n=6, P<0.05) are repressors of α and β sGC transcription, respectively, in HASMCs. Since CBF (1.7±0.1-fold, n=3, P<0.05) and NF-κB (1.8±0.2-fold, n=8, P<0.05) are also repressors in COS-7 cells, these TFs are likely to be key in the transcriptional control of α and β sGC genes. In contrast, CBF (8.0±0.3-fold, n=3, P<0.05), NF-AT (2.1±0.2-fold, n=3, P<0.05) and E2F (2.0±0.3-fold, n=3, These data therefore provide a systematic, comparative analysis of human α and β sGC promoter regulation in HASMCs (and COS-7 cells), thereby identifying potentially important factors regulating human sGC expression within a cell system relevant to cardiovascular physiology and pathology.
Kamisaki et al. 1986 J. Biol. Chem. , 261 , 7236 This work was supported by the Wellcome Trust. |
|