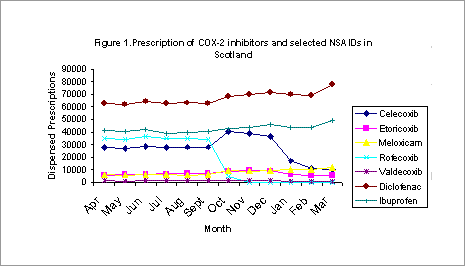
The effect of the withdrawal of rofecoxib on prescribing patterns of COX-2 inhibitors in scotland Introduction: In September 2004, rofecoxib was withdrawn by Merck pharmaceuticals after the adenomatous polyp prevention trial demonstrated an increase in cardiovascular events in patients with a history of colorectal adenomas who were randomised to receive rofecoxib, compared with those in the placebo group (Bresalier et al., 2005). Further concerns were raised regarding the cardiovascular safety of celecoxib and valdecoxib (Solomon et al., 2005; Nussmeier et al., 2005) . We wished to examine the effect of the withdrawal of rofecoxib on the prescription of other COX-2 inhibitors and other nonselective Non-Steroidal Anti-inflammatory Drugs (nsNSAIDs) in Scotland. Methods: Dispensed data for Scotland for the period April 2004-March 2005 were obtained for all COX-2 inhibitors (celecoxib, etoricoxib, meloxicam, rofecoxib, valdecoxib) and the most commonly prescribed nsNSAIDs (ibuprofen, diclofenac) from the Information Statistics Division (ISD) Scotland. Results: Prescribing trends for the COX-2 inhibitors and principle nsNSAIDS in Scotland are shown in Figure 1. The withdrawal of rofecoxib led to an initial increase in the prescription of celecoxib as prescribers presumably switched to this alternative agent. However, this rise was short-
lived, presumably as a result of concerns that the safety issue with rofecoxib might be a class effect. The prescription of celecoxib has since shown a significant further decline co-incident with safety concerns concerning this agent (Solomon et al., 2005). A parallel increase in the prescription of diclofenac and ibuprofen was also noted suggesting that prescribers are prescribing these medications as alternatives to COX-2 inhibitors. A further increase in the prescription of meloxicam and etodolac which also have COX-2 inhibitory properties was also noted. Conclusion: The debate on whether the cardiovascular adverse effects of COX-2 inhibitors are a class effect will continue. However our results suggest that whilst prescribers may have initially interpreted safety concerns regarding rofecoxib to be drug specific, our data provides evidence that prescribers have interpreted this effect to be class specific.
Bresalier et al.N Engl J Med 2005; 352(11):1092-102. Acknowledgements: We would like to acknowledge Information Statistics Division (ISD) Scotland for providing that prescription data on which these results were based. |