Proteolysis of annexin-A1 by proteinase 3 – an autoantigen in Wegener’s granulomatosis Annexin-A1 (Anx-A1) is an endogenous anti-inflammatory mediator with potent anti-migratory effects on polymorphonuclear leukocytes (PMN). Studies over the last 10 years have shown that N-terminal cleavage of Anx-A1 from the parental 37 kDa to a 33 kDa isoform precedes an inflammatory stimulus, and may serve to alter the biological function of ANXA1. Peptides encompassing the N-terminal mimic the effects of the full-length protein both in vitro and in vivo (Perretti et al., 1997). Proteinase 3 (PR3), a neutral serine protease present in the PMN, plays a key role in the development of Wegener’s granulomatosis (WG), a form of vasculitis characterized by an exaggerated PMN activation and their accumulation in the vascular wall. Given the importance of Anx-A1 in the regulation of PMN-endothelial cell interaction, we asked whether PR3 might be involved in Anx-A1 catabolism. Anx-A1 distribution in human resting PMN, PMN activated with tumour necrosis factor-α (TNF-α ; 10 ng/ml, 30 min), or rolled on TNF-α -activated human umbilical-vein endothelial cells was determined by western blotting, using a specific rabbit antibody (1:5000; Zymed). An Anx-A1 construct tagged with c-Myc and FLAG epitopes at the N- and C-terminal, respectively, was generated (pcDNA/MFA1) by standard molecular protocols, using the vector pcDNA3.1 (Invitrogen) for cloning. MFA1 protein was purified from cell extracts of pcDNA/MFA1-transfected HEK 293T cells and used as a substrate (30 min at 37°C) for in vitro cleavage by human PR3 (Elastin products). To examine Anx-A1 proteolysis by PR3 ex vivo, the human mast cell line HMC-1 over-expressing PR3 (HMC1/PR3), or the inactive PR3 mutant (HMC1/S207A) donated by Dr V Wikto-Sarsat, Inserm, Paris were transfected with pcDNA/MFA1.
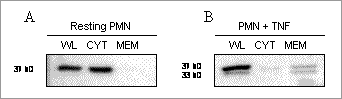
Figure 1. Western blot analysis of Anx-A1 distribution in PMN whole lysate (WL), cytosolic (CYT) or membrane (MEM) fractions. PMN were (A) unstimulated, or (B) activated with TNF-α (10 ng/ml, 30 min). PMN activation resulted in the redistribution and externalisation of N-terminal-intact Anx-A1 from cell cytosol to membrane surface (Figure 1, n=4). This was also visualised after PMN rolling (n=4). Anx-A1 externalization was accompanied by a proteolytic event leading to release the N-terminal-cleaved 33 kDa isoform. Specific N-terminal cleavage of MFA1 was demonstrated in vitro in the presence of purified PR3 (67 ± 5% reduction with 100 ng PR3, relative to control; n=4). In HMC1 cells over-expressing both the enzymatically inactive PR3 mutant and MFA1, extracts were found to contain only the N-terminal-intact isoform. In contrast, cells over-expressing PR3 and MFA1 contained neither c-Myc nor FLAG immunoreactivity (n=3), suggesting PR3-dependent proteolysis of Anx-A1. These results indicate that targeting Anx-A1 N-terminal proteolysis, might lead to a new class of drugs capable of blocking the excessive activation and adhesion of PMN to inflamed vessels, a characteristic of many chronic inflammatory diseases. Indications for Anx-A1 C-terminal cleavage were also obtained. Altogether these findings may have implications for PMN functions as detected in WG disease.
Perretti M (1997) Trends Pharmacol. Sci. 18,418-425. This work was supported by a PhD studentship (JRB XMSR) of the Research Advisory Board of Bart’s and The London Special Trustees. FDA is supported by the MRC, MP by the Arthritis Research Campaign, and RJF is a Principal Research Fellow of the Wellcome Trust. |