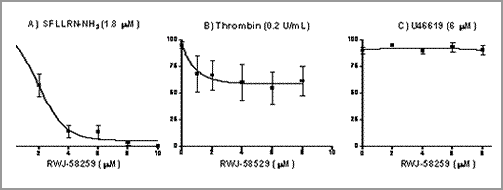
Thrombin induces protease-activated receptor independent human platelet aggregation Thrombin is central to the coagulation cascade as well as having important direct receptor mediated effects in the cardiovascular system inducing platelet aggregation and vascular inflammation. Protease activated receptors (PARs) are a novel group of G-protein coupled receptors characterized by a unique mechanism of activation. PAR-1 has been proposed as the principal receptor mediating the direct cellular effects of thrombin. We hypothesised that thrombin mediated platelet aggregation was entirely dependent on PAR-1 activation. Platelet aggregation in platelet rich plasma and washed platelets from citrated human blood (n=38) was measured using standard optical platelet aggregometry, in response to thrombin, the PAR-1 activating peptide SFLLRN-NH2, the PAR-4 activating peptide GYPGQV-NH2 and the thromboxane A2 agonist U46619, with and without the specific PAR-1 antagonist RWJ-58259 (αS-N-[(1S)-3-amino-1-[[(phenylmethyl)- amino]carbonyl]propyl]-alpha-[[[[[1-(2,6-dichlorophenyl)methyl]-3-(1-pyrrolidinylmethyl)-1H-indazol-6-yl]amino]carbonyl]amino]-3,4-difluorobenzenepropanamide) or the direct thrombin inhibitor, lepirudin. Thrombin, SFLLRN-NH2 and U46619 caused concentration-dependent platelet aggregation (n=8) but GYPGQV-NH2 had no effect (p=ns, n=6). RWJ-58259 (Figure 1), but not lepirudin, fully inhibited SFLLRN-NH2 (1.8 and 3 μM) induced aggregation (n=8, p<0.01; one-way ANOVA; IC50 =2.1 μM). Thrombin (0.2-1 U.ml -1, EC70 –EC100) mediated aggregation was only partially (up to 50%) inhibited by RWJ-58259 (n=8, p<0.01), but completely abolished by lepirudin (0.1-1 μg.ml-1; n=6, p<0.001).
We conclude that thrombin has significant PAR-1 independent effects on platelet aggregation that are not mediated by PAR-4. This has implications for the therapeutic potential of PAR-1 antagonists as antiplatelet and antithrombotic agents. |
|