Print version
Search Pub Med
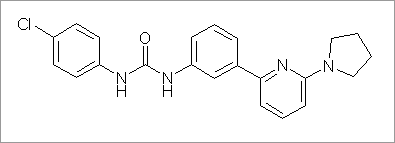
Characterisation of PSNCBAM-1, a novel allosteric antagonist of the cannabinoid type 1 receptor with in vivo efficacy in an acute rat feeding model Figure 1. Structure of PSNCBAM-1 Cannabinoid type 1 receptors (CB1) are widely distributed in the brain and in peripheral tissues such as fat, liver, and intestine. There is considerable interest in the development of CB1 antagonists for obesity treatment. Recently, the antagonist/inverse agonist SR141716A (rimonabant, AcompliaTM) has been shown to significantly reduce body weight, waist circumference and triglyceride levels in obese patients (Pi-Sunyer et al., 2006). Modulation of the CB1 receptor by compounds that bind to allosteric sites has recently been reported by Price et al. (2005). Here we describe the in vitro characterisation of a novel class of CB1 allosteric antagonists, typified by PSNCBAM-1 (Figure 1) and also provide the first demonstration of the in vivo efficacy of a CB1 allosteric antagonist in an acute rat feeding model. PSNCBAM-1 showed antagonism of CB1 by inhibiting the agonist effect of 100 nM CP55940 in a human CB1 (hCB1) yeast reporter assay with an IC50 value of 45.2 ± 7.5 nM (n=3, mean ± s.e.m.). Interestingly, PSNCBAM-1 was inactive in yeast cells expressing constitutively active hCB1, suggesting that the compound lacked inverse agonist properties. This was in contrast to SR141716A which reduced both CP55950 induced and constitutive CB1 signalling with IC50 values of 22.5 ± 7.3 nM and 4.8 ± 0.4 nM respectively. In competition binding assays, PSNCBAM-1 paradoxically increased the binding of [3H]CP55940 to HEK293-hCB1 cell membranes by 58 ± 9 %, indicating positive modulation of agonist binding. Partial inhibition of [3H]SR141716A binding was observed in similar experiments. Further characterisation in a mammalian functional assay confirmed the antagonist properties of the compound. PSNCBAM-1 inhibited CP55940 (50 nM) stimulated [35S] GTPγS binding in HEK293-hCB1 membranes with an IC50 value of 74.3 ± 12.7 nM, but not in HEK293-hCB2 membranes. A Schild analysis using this assay revealed the functional antagonism of PSNCBAM-1 to be non-competitive. Taking these data together, we conclude that PSNCBAM-1 acts as an allosteric antagonist of CB1. Furthermore, in acute food intake studies in freely feeding male Sprague-Dawley rats (391 - 607 g), PSNCBAM-1 (30 mg/kg, i.p. using 5 % propyleneglycol / 5 % Tween 80 / 90% saline as vehicle) caused a significant 48 ± 7 % reduction in food intake over 24 hours, as compared to 48 ± 3 % reduction by 10 mg/kg, i.p. SR141716A (n=6, mean ± s.e.m., P < 0.01, ANOVA and Dunnett’s test). Both PSNCBAM-1 and SR141716A were also found to decrease body weight significantly over 24 hours. In conclusion, these results provide evidence that novel specific allosteric antagonists of CB1, typified by PSNCBAM-1, may have the potential for use as anti-obesity agents.
Pi-Sunyer FX et al. (2006). JAMA. 295: 761-775. Price MR et al. (2005). Mol Pharmacol 68 : 1484-1495. |
|