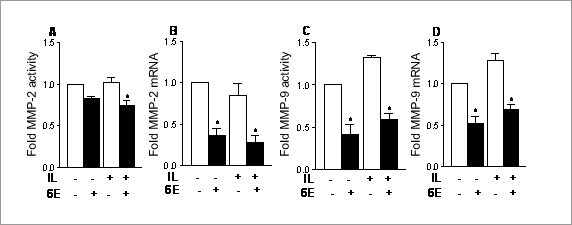
Inhibition of matrix metalloproteinase-2 & -9 activities by farnesoid X receptor in vascular smooth muscle cells The farnesoid X receptor (FXR; NR1H4) is a ligand-activated transcription factor that regulates bile acid and lipid homeostasis, and is expressed in the vasculature (Bishop-Bailey et al., 2004). The matrix metalloproteinases (MMP) are a family of Zn+-dependent endopeptidases that digest extracellular matrix. The gelatinases (MMP-2 and -9) regulate tissue remodelling, and are considered to be important in atherosclerotic plaque stability. We have therefore investigated whether FXR regulates the activities of MMP-2 and -9 in rat aortic smooth muscle cells (RASMC). RASMC culture and RT-PCR were as previously described (Bishop-Bailey et al., 2004). The FXR ligand, 6α-ethyl-chenodeoxycholic acid (6ECDCA; 30µM) or vehicle was added to confluent monolayers of RASMC 1h prior to 24h incubation with IL-1β (10ng/ml) or vehicle under serum-free conditions. The conditioned media were then removed for zymography (Singh et al., 2000) and quantification of the gelatinolytic activities by densitometry (Image J). Expression of mRNA for MMP-2 and -9 were assessed by RT-PCR (Burbridge et al., 2002). MMP-2 activity and mRNA (Figure 1A & B) were not changed by IL-1β, but the activity and expression of inducible MMP-9 (Figure 1C & D) were increased. 6ECDCA reduced both MMP-2 and -9 activities and gene expression in the presence or absence of IL-1β (Figure 1). FXR ligands down-regulate MMP-2 and -9 activities. FXR may therefore be a novel regulator of vascular remodelling and plaque stability.
Bishop-Bailey et al. (2004). Proc . Natl. Acad. Sci. USA. 101, 3668-73. This work was funded by the British Heart Foundation (FS/04/049/17115 and BS/02/002), and the Wellcome Trust (074361/Z/04/Z). |
|