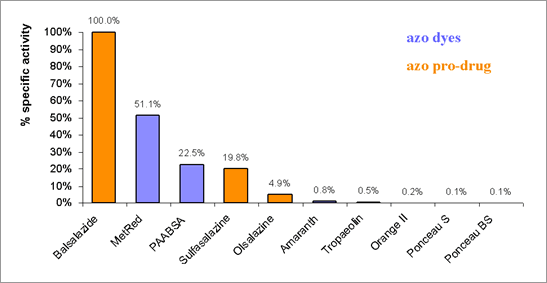
Molecular cloning, characterisation, and structural studies of an azoreductase from pseudomonas aeruginosa Azoreductase is a flavo-protein which catalyses the reductive cleavage of azo bonds (-N=N-) with a flavin-cofactor and NAD(P)H as electron donor. Bacterial azoreductases have been investigated in relation to their ability to break down environmentally polluting azo dyes (Nakanishi et al., 2001); however, bacterial azoreductases are also able to activate azo pro-drugs within the gut. The site-specific delivery of active drug molecules to the distal small bowel and colon is very important in the treatment of inflammatory bowel disease (IBD; Kinget et al., 1998). There is very little information about the specificities of gut bacterial azoreductases for azo pro-drugs; therefore, the aims of this study are to identify species in gut flora that express azoreductases, to characterise these enzymes biochemically and to identify whether azoreductases found in gut flora can activate azo pro-drugs. Azoreductases have been identified in a range of bacteria found in the gut including the pseudomonads using bioinformatics analysis and growing cultures of Pseudomonas aeruginosa PA01 were found to decolourise the azo dye, Methyl Red. Three azoreductase genes have been identified in the P. aeruginosa genome and one gene encoding for PA0785 has been heterologously overexpressed in E. coli BL21(DE3)pLysS. Recombinant PA0785 has been shown to activate the coloured azo pro-drugs, sulfasalazine and balsalazide, as shown in Figure 1. The enzyme activity has been determined spectrophotometrically by measuring the substrate concentration directly at a suitable wavelength. We have investigated the spectral properties of the recombinant protein and have determined the 3D structure of the protein by X-ray crystallography, which confirm that this enzyme is a flavo-protein, containing a tightly, but non-covalently bound FMN molecule. The purified recombinant PA0785 enzyme can activate azo pro-drugs that are used in the treatment of IBD. The 3D structure of the protein is similar to the structures of the azoreductase from E. coli (Ito et al., 2005) and a rat NAD(P)H:quinone reductase (Li et al., 1995). Further biochemical characterisations and structural studies of azoreductases present in gut flora will benefit our understanding of the activation of azo pro-drugs and their clinical efficacy.
Figure 1. Purified recombinant PA0785 azoreductase is able to metabolise coloured azo pro-drugs, Balsalazide, Sulfasalazine and Olsalazine, and a range of azo dyes as demonstrated by the loss of colour.
Nakanishi, M. et al., J. Biol. Chem., 2001; 276(49), 46394-46399. |
|