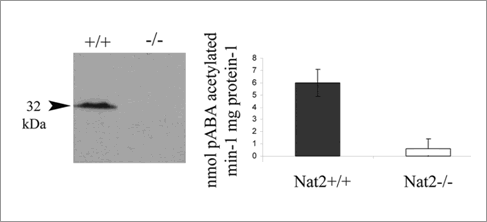
Murine arylamine N-acetyltransferase 2 (NAT2) expression in the developing neuroendocrine system Background: Arylamine N-acetyltransferase (NAT) genes in humans and in rodents encode drug metabolizing enzymes of clinical and toxological importance (Sim et al 2003), with one isoform (NAT1 in humans) significantly up-regulated in estrogen responsive breast tumours (Adam et al 2003). The presence of human NAT1 (and the murine equivalent mouse Nat2) protein in embryos and in a wide range of adult tissues suggests an endogenous role for this enzyme, distinct from its role in xenobiotic metabolism. AIMS: To identify the expression pattern of mouse Nat2 during development. Experimental: We have used a transgenic mouse model in which the murine Nat2 gene can be tracked by the presence of the lacZ transgene (Cornish et al 2003). The Nat2 knock-out/lacZ knock-in mouse strain was bred onto a C57Bl/6 strain to give a homogeneous genetic background. Nat2 gene expression was mapped using the expression of the lacZ marker gene in these Nat2-/- mice, and confirmed by immunohistochemistry using NAT2-specific antibodies in C57Bl6 mice (Stanley et al 1996) . The presence of functional NAT2 enzyme in tissues of Nat2+/+ embryonic and adult mice was demonstrated using a standard in vitro acetylation assay (Smelt et al 1997) and is shown immunohistochemically and enzymically in Fig.1 . Results: Detailed expression mapping shows that Nat2 is robustly expressed in sensory and effector components of the developing neuroendocrine system, specifically, in sensory epithelia and in epithelial placodes which generate visceral sensory neurons, as well as in the developing pituitary gland, sympathetic chain and urinogenital system. In vitro acetylation activity assays show that the NAT2 protein is enzymatically active.
Fig.1 Expression of murine Nat2 protein and activity in 11.5 day embryos. The Nat2-/- embryos lack the protein and acetylating activity.
Conclusions: The expression pattern supports an endogenous role for mouse Nat2 related to neuroendocrine function.
Sim et al. (2003) Biochemical Society Transactions. 31, 615-619 We thank the Wellcome Trust for funding. |
|