Effect of activated protein C(APC) in a murine model of acute lung injury (ALI)
APC is currently indicated for the treatment of severe sepsis, a condition associated with profound activation of the coagulation cascade. The mechanism of action of APC is currently uncertain as APC possesses both anti-coagulant and anti-inflammatory properties. The anti-inflammatory action of APC has recently been ascribed to activation of the thrombin receptor, proteinase-activated receptor-1 (PAR1), a receptor otherwise considered to mediate pro-inflammatory effects (Ruf, 2005). This paradox is thought to involve differential activation of PAR1 by thrombin or APC, leading to different cell signalling. In the present studies we investigated whether APC may be useful in single-organ failure such as ALI, in addition to systemic sepsis. In the current studies we have focussed our attention on the possible mechanism of action of APC, and whether PAR1 might be involved. Bacterial lipopolysaccharide (LPS) was administered intranasally to female BALB/c mice (6-8 weeks old) to induce an acute lung injury, and one hour later APC (or other drugs) were administered routes to mimic the clinical scenario of a patient admitted with an early inflammatory response. Mice were killed with an overdose of urethane (30 g.kg-1) 3 hours after LPS administration and bronchoalvelolar lavage (BAL) was performed to assess the inflammatory response in the airspaces. Differential counts (monocytes vs. neutrophils) were performed on cells retrieved in BAL fluid.
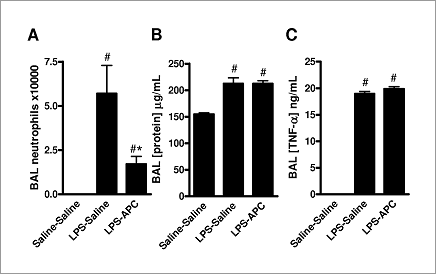
Figure 1: Anti-inflammatory effect of APC (0.05 mg.kg -1 i.v.). n=5 for each group. * indicates P<0.05 vs saline/saline. # indicates P<0.05 vs LPS/saline (ANOVA).
LPS administration elicited a neutrophil-rich inflammatory response, coinciding with an increase in BAL fluid levels of tumour necrosis factor-α (TNF- α) and an increase in BAL protein (Figure 1). Intravenous murine recombinant APC reduced the number of neutrophils found in BAL fluid, but had no effect on TNF-α or protein levels (Figure 1). By contrast, if APC was administered via the intranasal route, no anti-inflammatory effect was observed (14.9±3.7x104 BAL neutrophils vs. 10.1±1.7x104 control, P>0.05). The anti-inflammatory effect of APC was mimicked by a low dose of the PAR1 agonist peptide TFLLR (1 μmole.kg-1; 13.2±1.6x104 BAL neutrophils vs. 7.3±6x104 control, P=0.03) whereas higher doses of TFLLR (20 μmole.kg-1) had no effect (6.5±1.6x104 BAL neutrophils vs. 10.3±2.0x104 control, P>0.05). Dose-ranging studies indicate that at 0.05 mg.kg-1, APC is protective, but is ineffective at higher (0.1-0.5mg.kg-1) or lower doses (0.001-0.025 mg.kg-1). These results suggest that intravenously infused, but not inhaled, APC might be useful in the treatment of ALI. The similar effect of a low concentration of a PAR1 agonist suggests that PAR1 may mediate the protective effect of APC, while the dose-response data indicate that APC has a narrow therapeutic range, and higher doses possibly mimic the pro-inflammatory effects of thrombin at PAR1.
Ruf, W. J. Thromb. Haemost., 3, 1912-1914 |
|