Print version
Search Pub Med
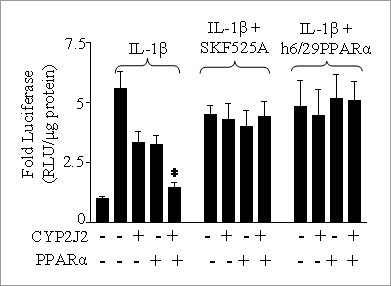
Cytochrome P450 2J2 inhibits nuclear factor-κB through activation of peroxisome proliferator-activated receptor-α. Peroxisome proliferator-activated receptor (PPAR)-α is a nuclear receptor that has anti-inflammatory properties (Bishop-Bailey, 2000). Cytochrome P450 (CYP)2J2 is an epoxygenase that is anti-inflammatory in the cardiovascular system (Node et al., 1999). We have recently shown that CYP2J2 activates PPAR a (Wray et al., 2003). Both CYP2J2 and PPAR a inhibit the pro-inflammatory transcription factor nuclear factor(NF)- kB. We have investigated whether CYP2J2 inhibits NF kB through its activation of PPAR a. Human embryonic kidney cell (HEK)293 culture, transfections, and luciferase reporter gene assays were as previously described (Wray et al., 2003). Cells were transfected with combinations (0.5µg of each) of pNFκB-luc NFκB luciferase reporter gene, pcDNA-CYP2J2, mPPAR IL-1β induced NFκB reporter gene activation was completely inhibited by the combination of PPARα and CYP2J2. This PPAR a and CYP2J2 inhibited NF kB reporter gene activation was reversed by inhibition of either CYP2J2 or PPARα by SKF525A or co-transfection with h6/29PPAR a, respectively (Figure 1)
Figure 1. CYP2J2 and PPARα inhibit IL-1 b induced NFκB reporter gene activaiton. NFκB activity was measured in HEK293 cells transfected with pNFκB-luc, pcDNA, pcDNA-CYP2J2, mPPARα, and/or h6/29PPARα. Cells were treated with IL-1β (10ng/ml) for 16h, and/or a 1h pre-treatment with SKF525A (30μM). Data represents n=9 from n=3 experiments. * P<0.001 One-way ANOVA with Bonferroni post test, significant difference from IL-1 β pcDNA control.
CYP2J2 inhibits NFκB reporter gene responses by activating PPAR a . PPAR a therefore represent a novel endogenous pathway by which CYP2J2 can exert its anti-inflammatory/ anti-proliferative effects.
Bishop-Bailey, D. (2000). Br. J. Pharmacol. 129 , 823-34. Funded by the British Heart Foundation (FS/04/075 and BS/02/002). |
|