074P Brighton
Winter Meeting December 2007 |
The Effect Of Novel Xenoestrogens On The Maxi-K Potassium Channel
Jacqueline Maher1, Natasha Tisovszky1, Christy Hunter1, Heidi de Wet2, Jonathan Lippiat3, Marcus Allen1
1University of Brighton, Sussex, United Kingdom, 2University of Oxford, Oxford, United Kingdom, 3University of Leeds, Yorkshire, United Kingdom
The Maxi-K (BK) channel is a large conductance potassium (K+) channel that is Ca2+ and voltage dependent. Activation of this channel leads to smooth muscle relaxation, and could potentially be targeted to treat hypertension. Oestrogens and xenoestrogens have been shown to have no effect on the BK channel when only α-subunits are present. However they lead to increased activation with addition of the regulatory β1-subunit (De Wet, et al 2006). The aim of our research was to further test this effect with the use of oestrone, and its novel derivatives (DME-oestrone, QuatDME-oestrone).
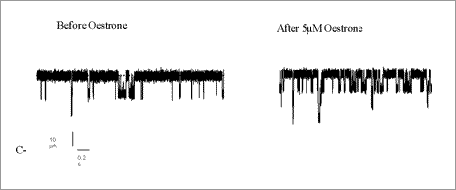
Maxi-K protein(s) was incorporated into a planar lipid bilayer and channel conductance was recorded under voltage clamp conditions. Single channel currents were measured at holding voltages of 50mV. Lipid bilayers were painted across a 0.25mm diameter polystyrene cup which separated two symmetrical KCl (150mM) Ringer’s, called cis and trans. The membrane proteins (containing the subunits) and the drugs were added to the cis side. Recordings (0-450s) were analyzed using WinEDR software to determine NPo (open probability). These were then statistically analyzed using a Wilcoxon matched pair test, and significance was indicated at p<0.05.
Preliminary data analysis suggests that adding 5μM oestrone (NPo=0.739±0.23 before; NPo=0.636±0.21 after; n=7, p>0.05), 5μM DME-oestrone (NPo=0.822±0.16 before; NPo=0.812±0.17 after; n=8, p>0.05), or 5μM QuatDME-oestrone (NPo=0.668±0.12 before; NPo=0.6±0.12 after; n=7, p>0.05), does not increase the NPo of BK channels if only the α-subunit is present. Oestrone (5μM) however, increased NPo in the presence of the β1-subunit (NPo=0.101±0.02 before; NPo=0.181±0.05 after; n=7, p<0.02) (see below). 5μM DME-oestrone (NPo=0.38±0.08 before; NPo=0.65±0.26 after; n=7, p>0.05) and 5μM QuatDME-oestrone (NPo=0.252±0.09 before; NPo=0.246±0.13 after; n=7, p>0.05) has no significant effect on NPo even in the presence of the β1-subunit.
Our study suggest that an exchangeable proton on the phenolic A ring is important if steroidal oestrogens are going to retain the ability to activate BK channel in the presence of the β1-subunit.
De Wet, H. et al.,(2006) Molecular Membrane Biology, Vol. 23:5, pp. 420-429
|



