122P Brighton
Winter Meeting December 2007 |
Mechanisms underlying the role of TRPV1-dependent responses in sepsis
Elizabeth S. Fernandes, Julie Keeble, Lihuan Liang, Yanira R. Vasquez, Susan D. Brain
King’s College London, London, United Kingdom
Sepsis is an infective disease associated with inflammation and little is known about the protective innate mechanisms against the onset of sepsis (Remick, 2007). Recently, TRPV1 was suggested to exert a protective role on lipolysaccharide (LPS)-induced sepsis (Clark et al., 2007). TRPV1 deletion worsened sepsis with increased nitric oxide and tumour necrosis factor (TNF)-α levels in the peritoneal cavity. We now investigate possible immune mechanisms underlying the protective role of TRPV1 on sepsis.
For this purpose, we used a murine model of sepsis induced by LPS injection. All procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986 and mice were kept in a climatically controlled environment and given food and water ad libitum. In all studies male CD1 mice were utilised at 8 weeks old (N=5-8). Animals received an intraperitoneal (i.p.) injection of saline 0.9% containing 11.25 106 endotoxin units/kg (10 ml/kg) or saline (control animals). Cell influx and cytokine release were evaluated in the peritoneal lavage at 4 h after LPS treatment. TRPV1 protective role was assessed by using the selective TRPV1 receptor antagonist SB366791 (500 μg/kg, i.p.) 30 min pre-LPS injection. Total and differential cell counts were carried out (Pitchford et al., 2003).Cytokine release was assessed by means of MSD ® immunoassay (Meso Scale Discovery,USA).
LPS led to a significant increase in neutrophils into the peritoneal cavity when compared to saline group (Table 1). Neutrophil migration was higher in animals pre-treated with the TRPV1 antagonist SB366791 (Table 1). Also, LPS caused the release of cytokines into the peritoneal cavity (Table 1). SB366791 treatment induces the release of high levels of TNFα and IL-12 (Table 1).
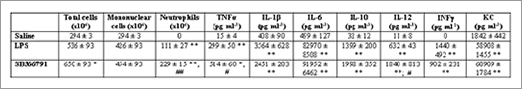
Table 1. Effect of the TRPV1 antagonist on cellular migration and cytokine release induced by LPS intraperitoneal injection into the peritoneal cavity. *P<0.05; **P<0.01 Significant different from Saline. #P<0.05; ##P<0.01 Significant different from LPS.
TRPV1 activation seems to be a key event controlling LPS-induced neutrophil migration as well as TNFα and IL-12 release. This data bring new ideas about the importance of TRPV1 in inflammatory conditions, but the exact mechanism remains to be further investigated.
Clark,N. et al. (2007). FASEB J. Available at: http://www.fasebj.org/cgi/rapidpdf/fj.06-7460comv1.pdf
Remick, DG. (2007). Am. J. Pathol., 170:1435-1444.
Pitchford, S.C. et al. (2003). J. Allergy Clin. Immunol., 112:109-118
E.S.F is funded by the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico-CNPq, Brazil
|


