064P Brighton
Winter Meeting December 2007 |
Polychlorinated biphenyl-induced endothelial cell dysfunction is mediated by poly (ADP-ribose) polymerase activation
Simon Helyar1, Jon Mabley2
1Brighton & Sussex Medical School, Brighton, East Sussex, United Kingdom, 2University of Brighton, Brighton, East Sussex, United Kingdom
The persistent environmental contaminants polychlorinated biphenyls (PCB) have been linked to an increased incidence of cardiovascular disease including atherosclerosis. PCBs cause endothelial cell dysfunction through increased oxidative stress via interaction with the Ah receptor and activation of cytochrome P450 1 subfamily (CYP1A1). Increased oxidative stress in endothelial cells has been shown to cause DNA single strand breaks and over-activate the DNA repair enzyme poly (ADP-ribose) polymerase (PARP) with subsequent decrease in cellular levels of high energy phosphates and NAD resulting in cell dysfunction.
The aim of this study was to examine the effect of PCB 104 on acetylcholine-mediated aortic ring relaxation and endothelial cell viability. In addition we investigated the role of CYP1A1 and PARP activation in PCB-mediated endothelial cell dysfunction using specific antagonists.
Thoracic aorta isolated from male Sprague-Dawley rats (180-220g) was cut into rings and exposed to PCB 104 ex vivo for 4 or 6h at 1-10μM. Following this incubation the aortic rings were mounted in organ baths filled with warmed and oxygenated Krebs solution with isometric tension measured with isometric transducers and digitized using PowerLab. The contractile response (to phenylepherine) and relaxant response (to acetylcholine) was determined. Endothelial cell viability was determined using a mouse endothelial cell line exposed to PCB 104 for 24 or 48h followed by an MTT assay
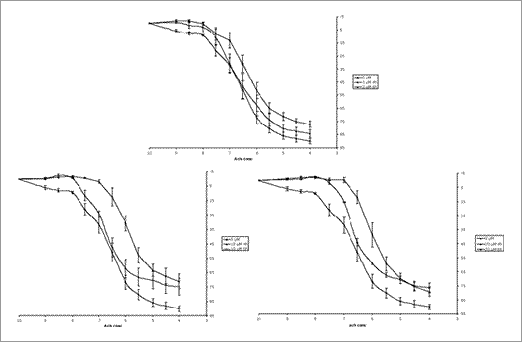
Fig 1. Demonstrates that exposure of rat aortic rings to increasing physiological concentrations (3, 10 or 20μM) of PCB 104 cause endothelial cell dysfunction and that this dysfunction is also dependent on time of exposure.
The concentration of acetylcholine required for 50% relaxation was 3x10-8M in control rings while exposure for 4h to 10μM PCB 104 significantly increased this to 1.5x10-7M (p<0.05 by analysis of variance with Bonferroni’s correction). Treatment with 3μM PJ-34 (a PARP inhibitor) protects the aortic ring function with the concentration of acetylcholine causing 50% relaxation being 5x10-8M (p<0.05 vs. PCB 104 alone), additionally treatment with 0.3μM α-naphthoflavone (a CYP1A1 inhibitor) also protected with 4x10-8M acetylcholine causing 50% relaxation. Exposure of mouse endothelial cells to PCB 104 caused a dose and time dependent decrease in cell viability, an effect which is again attenuated by PARP inhibition either pharmacologically using PJ-34 or by gene deletion.
In conclusion, 4 or 6h exposure to PCB 104 causes endothelial cell dysfunction via activation of CYP1A1 and PARP. Long term exposure of endothelial cells to PCB 104 causes dose dependent loss of cell viability an effect also mediated by PARP activation. Therapeutic PARP inhibitors may prove effective treatments for individuals exposed to PCB 104.
|