081P Brighton
Winter Meeting December 2007 |
The EP3 receptor is responsible for PGE2-induced sensory nerve activity: implications for asthma therapy
Sarah Maher, Mark A. Birrell, Maria G. Belvisi
Imperial College London, London, United Kingdom
Prostaglandin E2 (PGE2) may provide a novel approach for the treatment of inflammatory airway diseases as it has been shown to confer both bronchodilator and anti-inflammatory activity in asthmatic subjects (Melillo et al., 1994; Gauvreau et al., 1999). However, the development of prostanoid agonists for the treatment of airway inflammatory diseases has been hindered as PGE2 can cause irritancy of the upper airway resulting in a reflex cough. The aim of this study is to investigate which of the 9 prostanoid receptors (EP1-4, DP1/2, FP, IP or TP) are responsible for the airway irritancy and cough. The cough reflex is under the control of sensory afferent nerve fibres that innervate the lungs via the vagus nerve. Using our in vitro model of vagal sensory nerve depolarisation (mV) (Birrell et al., 2002), we investigated the prostanoid receptor subtype(s) responsible for the PGE2-induced depolarisation of the vagus nerve, and hence the receptor that may be involved in this tussive side effect, using selective prostanoid antagonists and prostanoid receptor knock-out (KO) mice.
The effect of prostanoid receptor antagonists was investigated on the depolarisation induced by PGE2 (10μM) in mouse (male C57bl/6j, 18-20g) and guinea-pig (male Dunkin Hartley, 300-350g) isolated vagal nerve preparations. A significant inhibition was determined using a paired t-test (n=4-6). Antagonists at the EP1, EP2, EP4, DP, FP, IP and TP receptors had no effect on the response to PGE2 whereas an EP3 receptor antagonist (0.2μM) significantly inhibited PGE2-induced depolarisation of the vagus nerve in the guinea-pig and mouse by 79.5 ± 8.8% (p<0.01) and 64.8 ± 2.7% (p<0.01) respectively. Using vagal nerves from prostanoid receptor KO mice (male, all C57bl/6j except EP4; 129Ola/C57bl6j, 18-20g), the response to PGE2 (10μM) was significantly reduced in EP3KO mice (Figure 1) (n=4-6, **p<0.01; Kruskal-Wallis, Dunns post-hoc test).
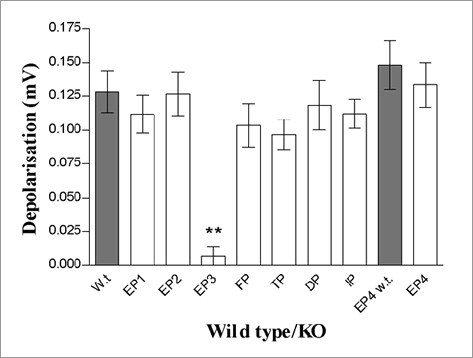
Figure 1: Response to PGE2 in KO mice
In conclusion, PGE2-induced depolarisation of mice and guinea-pig vagus nerves can be inhibited by the EP3 antagonist and EP3KO mice have a reduced depolarisation response to PGE2. This suggests that the EP3 receptor mediates PGE2-induced sensory nerve activity and could be involved in the PGE2-induced cough. If this is the case, and the receptor that is responsible for the anti-inflammatory and bronchodilator effects of PGE2 is different, then a therapy for asthma could be developed that is devoid of the tussive side effects.
Birrell et al. (2002) British Journal of Pharmacology, 136, p620-628
Gauvreau et al. (1999) American Journal of Respiratory and Critical Care Medicine, 159, p31-36
Melillo et al. (1994) American Journal of Respiratory and Critical Care Medicine, 149, p1138-1141
|