141P Brighton
Winter Meeting December 2007 |
IL-1beta plays a crucial role in TNFalpha-induced thermal hyperalgesia, in a model of inflammatory pain
Fiona Russell, Susan Brain. King's College London, London, United Kingdom.
TNFalpha-induces bilateral thermal hyperalgesia (increased sensitivity to heat) that is TRPV1-dependent in mice and relies partially on prostaglandins (Russell et al., 2005; Russell et al., 2006). TNFalpha induces the release of further cytokines, in particular, the pro-inflammatory cytokine, IL-1beta. The aim of this study was to determine whether TNFalpha-induced bilateral hyperalgesia is dependent on IL-1beta release.
Female CD1 mice (25-30g) were given intraplantar injections (i.pl.) of TNFalpha (10pmol/50microl) and Tyrode (as vehicle, contralateral paw; 50microl). Mice were culled 1h after injection, paws were removed and homogenised. Plasma was also collected. IL-1beta levels in paw homogenates and plasma were measured using an MSD cytokine assay (Mouse Pro-inflammatory 7-Plex kit, Meso-Scale Discovery). A second group of mice received TNFalpha (10pmol i.pl.) plus IL-1 receptor antagonist (IL-1ra; 500ng) in the ipsilateral paw and Tyrode plus corresponding amounts of IL-1ra vehicle (0.1% BSA saline) in the contralateral paw. A third group received i.pl. TNFalpha (10pmol i.pl.) plus IL-1ra vehicle in ipsilateral paw and Tyrode plus IL-1ra (500ng) in contralateral paw. Thermal hyperalgesic thresholds were measured using the Hargreaves technique before and 1h after injection (modified as Keeble et al., 2005).Mean of triplicate values was taken as paw withdrawal latency. Results were mean ± s.e.m. and statistical analysis performed using paired or unpaired t-tests as appropriate.
IL-beta levels were raised in both ipsilateral and contralateral paws (31 ± 4 and 22 ± 3 pg/mg protein, respectively) despite undetectable plasma levels. IL-1ra co-injected with TNFalpha prevented the bilateral hyperalgesia at 1h (Figure 1A), whereas IL-1ra co-injected with Tyrode prevented the hyperalgesia in that paw but hyperalgesia was still present in the TNFalpha-treated paw (Figure 1B).
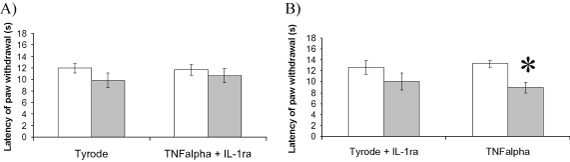
Figure 1. Thermal hyperalgesic thresholds before and 1h post- injection of A) TNFalpha plus IL-1ra (ipsilateral paw) and Tyrode (contralateal paw) B) TNFalpha ipsilateral paw) and Tyrode plus IL-1ra (contralateral paw). n=8-10,*p<0.05 compared to baseline value in same paw.
The presence of biologically active IL-1beta is crucial in both paws for the development of TNFalpha-induced thermal hyperalgesia. Furthermore, ipsilateral IL-1beta is necessary for the development of the contralateral hyperalgesia, possibly through causing sensory nerve sensitisation.
Keeble et al., (2005) Arthritis Rheum. 52, 3248-56
Russell,F.A. et al., (2005).Proceedings of the British Pharmacological Society at: http://www.pa2online.org/abstracts/Vol3Issue2abst115P.pdf
Russell,F.A. et al., (2006).Proceedings of the British Pharmacological Society at: http://www.pa2online.org/abstracts/1Vol4Issue2abst033P.pdf
F.R is funded by a BBSRC/Pfizer CASE studentship.
|


