009P Brighton
Winter Meeting December 2008 |
Subunit arrangements in heteromeric 5-HT3AB receptors
Andrew Thompson, Kerry Price, Sarah Lummis
Cambridge University, Cambridge, UK
5-HT3 receptors (5-HT3R) belong to the Cys-loop family of ligand-gated ion channels that are responsible for fast synaptic neurotransmission. The family also includes nACh, glycine and GABAA Rs, all of which are pentameric. To date five 5-HT3R subunits have been identified (A - E). A subunits can form functional homomeric Rs, but the other subunits must combine with A to form heteromeric complexes that alter the electrical properties of the R (Niesler et al., 2007; Davies et al., 1999). Atomic force microscopy of 5-HT3ABR has indicated that the arrangement of subunits is BBABA, but given that the ligand binding site is located between two adjacent subunits (principal & complementary), it does not explain why the pharmacology of the homomeric (AA interface) and heteromeric (AB, BA or BB interfaces) Rs are identical (Barrera et al., 2001; Brady et al., 2001). Here we use MTSEA modification of cysteine mutants to probe the arrangement of A and B subunits.
Human 5-HT3A (accession number: P46098) subunit cDNA was cloned into pGEMHE for Xenopus oocyte expression. cRNA was in vitro transcribed from linearised plasmid cDNA template using the mMessage mMachine T7 Transcription kit. Oocytes were injected with 5 ng cRNA, and currents recorded 1 - 4 days later. Cysteine mutants were generated using QuikChange® site-directed mutagenesis.
Cysteine substitutions were made within binding loops A (L121, I134), B (T176), D (R87) and E (Y138, Q146) of the A subunit, and corresponding residues of the B subunit. Effects of MTSEA (2 mM, 2 min) on concentration-response curves were measured. After MTSEA modification, Amut and AmutB combinations combinations showed changes in EC50 values, and maximal currents were either increased (AI134C, AI134CB), decreased (AR87, AR87B, AL121C, AL121CB, AQ146C, AQ146CB), or blocked (AT176C, AT176CB). In contrast, all ABmut were unaffected by MTSEA (Fig 1). AY138C and AY138CB were non-functional, but the ABY136C counterpart was functional and unaffected by MTSEA.
Affects of substitutions in both the principal and complementary face of the binding site are only observed when the A subunit is mutated, suggesting that the ligand binds at an A - A interface. These data may also explain why the pharmacology of 5-HT3AR and 5-HT3ABR competitive antagonists are identical.
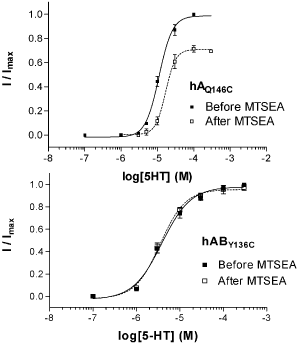
Fig 1. Example data showing the effect of MTSEA on 5-HT concentration-response curves. Mean ± SEM, n = 3 – 5.
Barrera, N.P. et al., (2005) PNAS USA. 102, 12595-12600.
Brady, C.A. et al., (2001) Neuropharmacol. 41, 282-284.
Davies, P. A. et al., (1999) Nature 397, 359-363.
Niesler, B. et al., (2007) Mol Pharmacol. 72, 8-17.
|


