Print version
Search Pub Med
Characterisation of the metabolic response to hypothalamic neuropeptides involved in energy balance Obesity is a leading cause of morbidity and premature mortality in the UK and costs the NHS in excess of £2 billion per year. The hypothalamus is an important CNS region in the regulation of energy balance. Understanding the mechanisms involved in the regulation of energy balance is important for the identification of novel targets for anti-obesity therapies. The hypothalamic control of energy balance is regulated by a complex network of neuropeptide-releasing neurones. Whilst the effect of these neuropeptides on individual aspects of energy homeostasis has been studied, the coordinated response of these effects has not been comprehensively investigated. The Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments) allows continuous and simultaneous measurement of a number of metabolic parameters including food intake, activity and energy expenditure 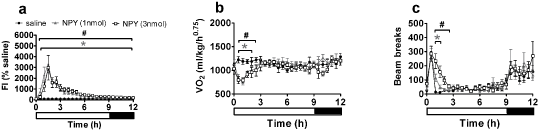
Figure 1: Effect of single ICV injection of NPY (1 or 3nmol) on (a) food intake, (b) oxygen consumption and (c) activity in male Wistar rats. *p< 0.05 1nmol NPY; #p<0.05 3nmol NPY vs saline (n = 8 per group). Black bar at bottom indicates dark phase. In contrast, orexin-A increased both feeding and oxygen consumption, consistent with an observed increase in activity. The potent anorexigenic effects of CRF were accompanied by a prolonged increase in activity whilst NMU injection resulted in significant but short-lasting effects on food intake, activity and oxygen consumption. Interestingly, α-MSH injection resulted in significant increases in activity and oxygen consumption with both doses whilst a reduction in food intake was only seen following administration of the 3nmol dose. This study furthers our understanding of these neuropeptides by generating a temporal and quantitative analysis of their effects on metabolic parameters. Furthermore, the CLAMS is a useful tool for characterising the metabolic response to pharmacological agents for the treatment of obesity |
|

